National University of Singapore (NUS) chemists have made a groundbreaking advancement in the field of artificial photosynthesis with the development of hexavalent photocatalytic covalent organic frameworks (COFs). These COFs mimic natural photosynthesis to produce hydrogen peroxide (H2O2), a crucial industrial chemical. The traditional method of H2O2 production involves using anthraquinone as a catalyst, which requires significant energy, costly noble metal catalysts, high-pressure hydrogen gas, and hazardous solvents. The artificial photosynthesis process developed by the NUS research team offers a sustainable and promising alternative to the conventional anthraquinone method by utilizing sunlight as an energy source, and abundant water and air as feedstocks.
One of the major challenges faced by artificial photosynthesis for H2O2 production is the inefficient generation of charge carriers and fast charge recombination, leading to lower efficiency. Additionally, the limited number of available catalytic sites results in low productivity, and the lack of efficient delivery of charges and reactants to the catalytic sites causes sluggish reaction kinetics. To address these challenges, Professor Donglin Jiang and his team from the Department of Chemistry at NUS devised a new strategy involving the development of hexavalent photocatalytic COFs through the systematic design of the π skeletons and pores.
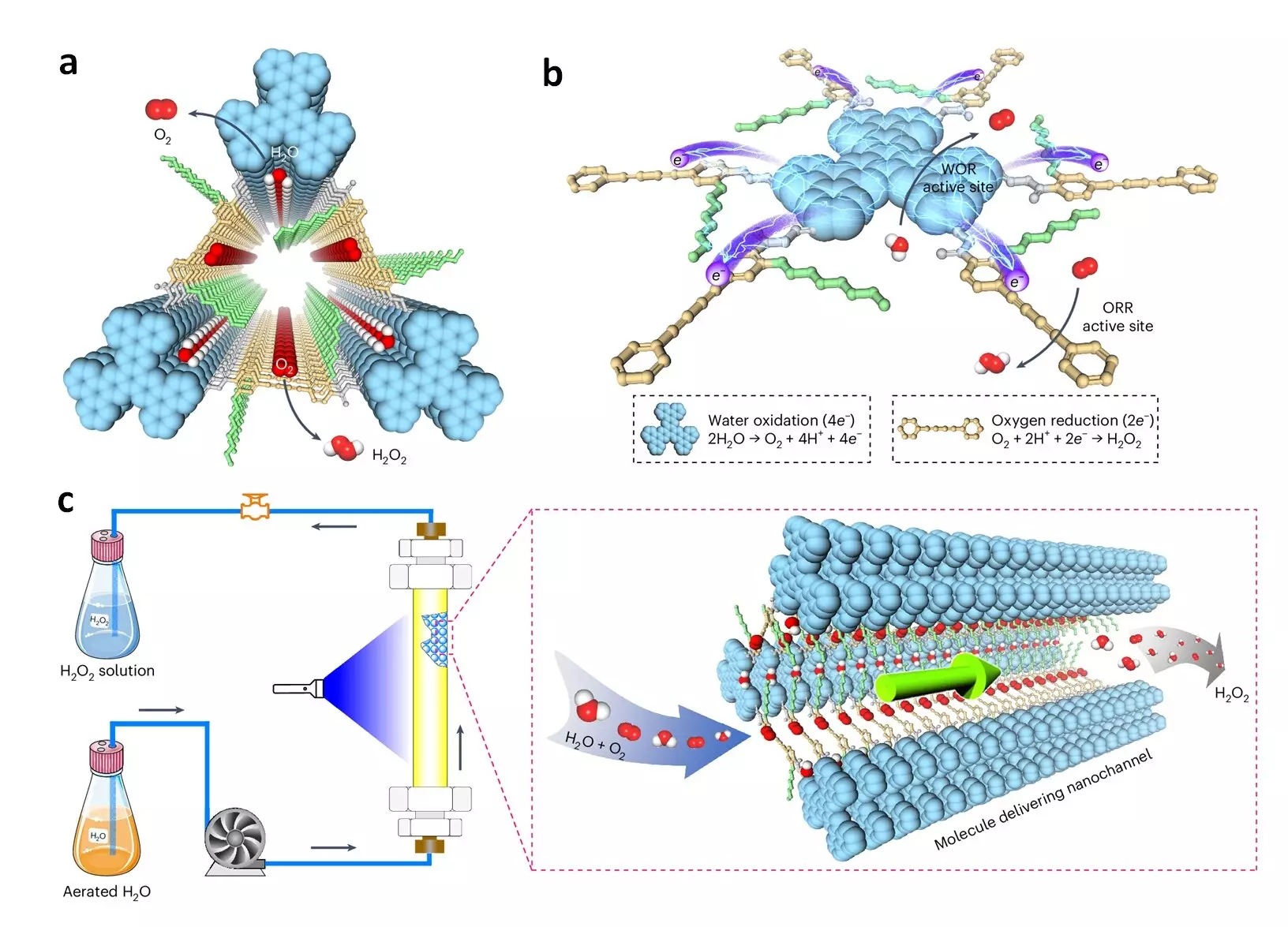
The photocatalytic COFs developed by the research team are porous, crystalline materials constructed from organic molecules linked by strong covalent bonds. Their flexibility makes them an ideal platform for constructing photocatalysts. The researchers introduced a new type of donor-alt-acceptor framework photocatalysts that transform into catalytic scaffolds with dense catalytic sites for oxygen reduction and water oxidation upon irradiation. These photocatalysts feature spatially segregated donor and acceptor columns for holes and electrons separation, preventing charge recombination and facilitating rapid charge transport. Furthermore, the pore walls of the photocatalytic COFs are engineered to be hydrophilic, enabling water and dissolved oxygen to reach the catalytic sites through capillary action in the 1-dimensional channel.
The hexavalent photocatalytic COFs have demonstrated remarkable performance metrics as a photocatalyst for H2O2 production, utilizing only water, air, and light. They achieve a production rate of 7.2 mmol g−1 h−1, an optimal apparent quantum yield of 18.0%, and a solar-to-chemical conversion efficiency of 0.91% in bath reactors. When integrated into flow reactors, these COFs sustainably produce over 15 liters of pure H2O2 solution under ambient conditions, showcasing operational stability over a two-week period. Professor Jiang expressed, “This work signifies nearly two decades of our collective endeavors in the realm of COFs, resulting in the development of innovative photocatalysts that effectively tackle the dual challenges of delivering charges and mass to catalytic sites efficiently.”
The development of hexavalent photocatalytic COFs by NUS chemists represents a significant breakthrough in the field of artificial photosynthesis for hydrogen peroxide production. By addressing key challenges and utilizing innovative design strategies, these COFs offer a sustainable and efficient alternative to traditional H2O2 production methods. This research paves the way for further advancements in the field of photocatalysis and renewable energy technologies.

Leave a Reply