Water is a substance that defies expectations in many ways – from its unusually high freezing and boiling points to its lower density as a solid compared to a liquid. These unique properties can be traced back to the presence of weak hydrogen bonds between water molecules. The attraction between the slightly negative oxygen and slightly positive hydrogen atoms leads to the formation of these bonds.
While most water molecules form hydrogen bonds with their neighbors, those on the surface experience a different interaction. The oxygen-hydrogen bonds of surface water molecules are affected by the interface with air, making it challenging to study their behavior. For years, researchers have struggled to understand how these molecules relax after being stretched due to the difficulty in isolating their signals.
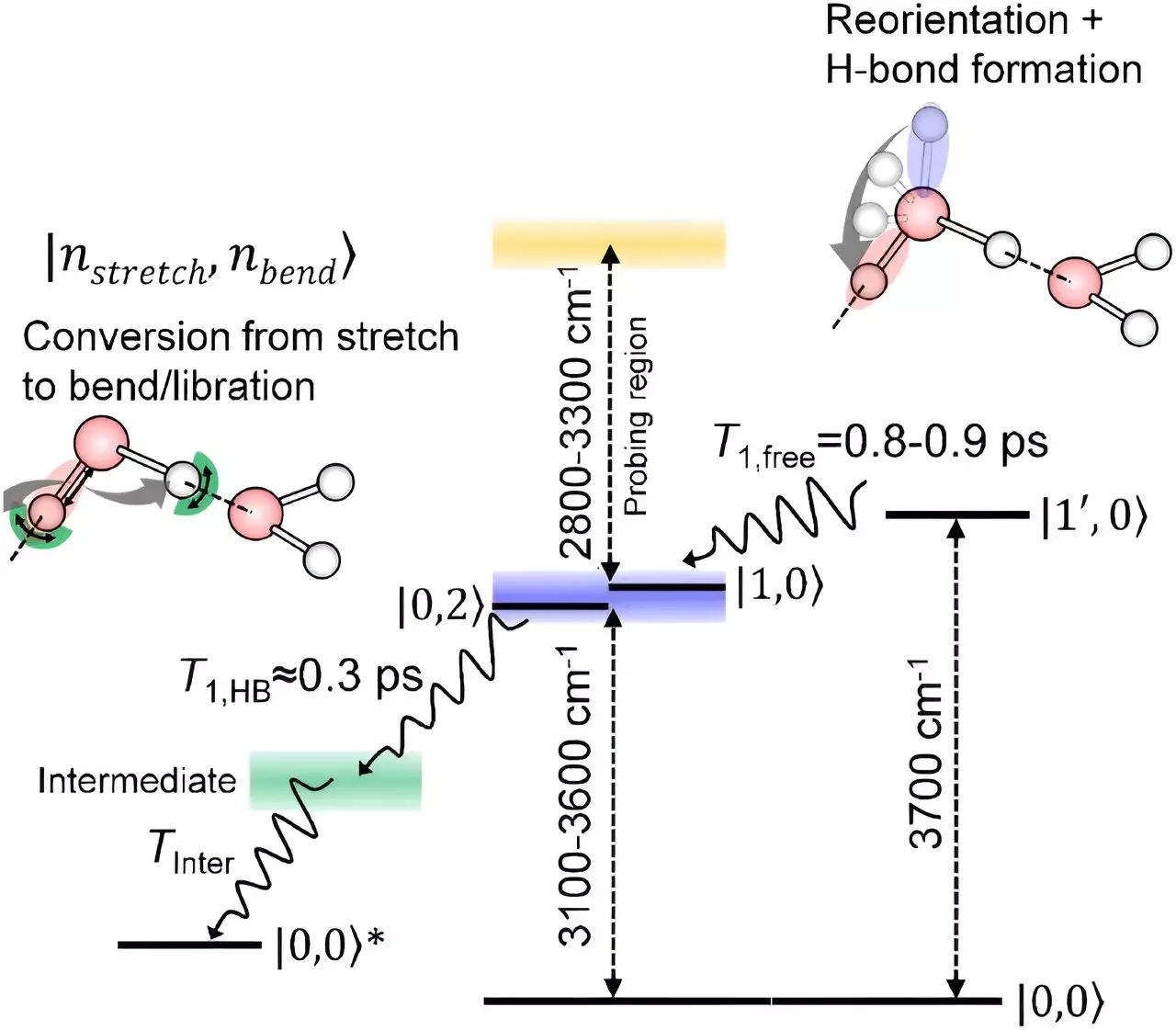
A team of scientists at RIKEN, led by Tahei Tahara, has made significant progress in unraveling the mysteries of water molecules at the interface with air. By developing advanced spectroscopy techniques, they were able to detect how the oxygen-hydrogen bonds of surface water molecules relax. Using an innovative approach based on infrared spectroscopy, the team found that these bonds rotate before eventually relaxing in a similar manner to molecules in the body of the liquid.
The findings from the study provide a more comprehensive picture of how water molecules at the interface with air lose their energy. Contrary to previous beliefs, the relaxation process of oxygen-hydrogen bonds at the surface is similar to that of molecules inside the liquid. This insight sheds light on the complex behavior of water molecules and enhances our understanding of processes that take place at water surfaces.
Building on their success, Tahara and his team plan to further explore the applications of their spectroscopic technique. They aim to investigate chemical reactions that occur at the interface of water, bringing new insights into the dynamic interactions of water molecules with their surroundings. The ongoing research holds the promise of uncovering more secrets about the behavior of water at interfaces, paving the way for future discoveries in the field of molecular interactions.

Leave a Reply