Calcite, a crystalline form of calcium carbonate, is a common mineral found in limestone and marble. Its rhombohedral shape gives it a unique appearance under a microscope. When synthesized, calcite plays a crucial role in transforming carbon dioxide into solid carbonate, making it an essential element in the fight against climate change.
The study of calcite has broad relevance due to its various properties and applications. Recent research has shown that defects in calcite can be controlled based on the synthesis method, leading to alterations in its reactivity. These defects can impact calcite’s ability to absorb harmful substances, affect its mechanical strength, and influence the development of more durable materials and improved catalysts for industrial processes.
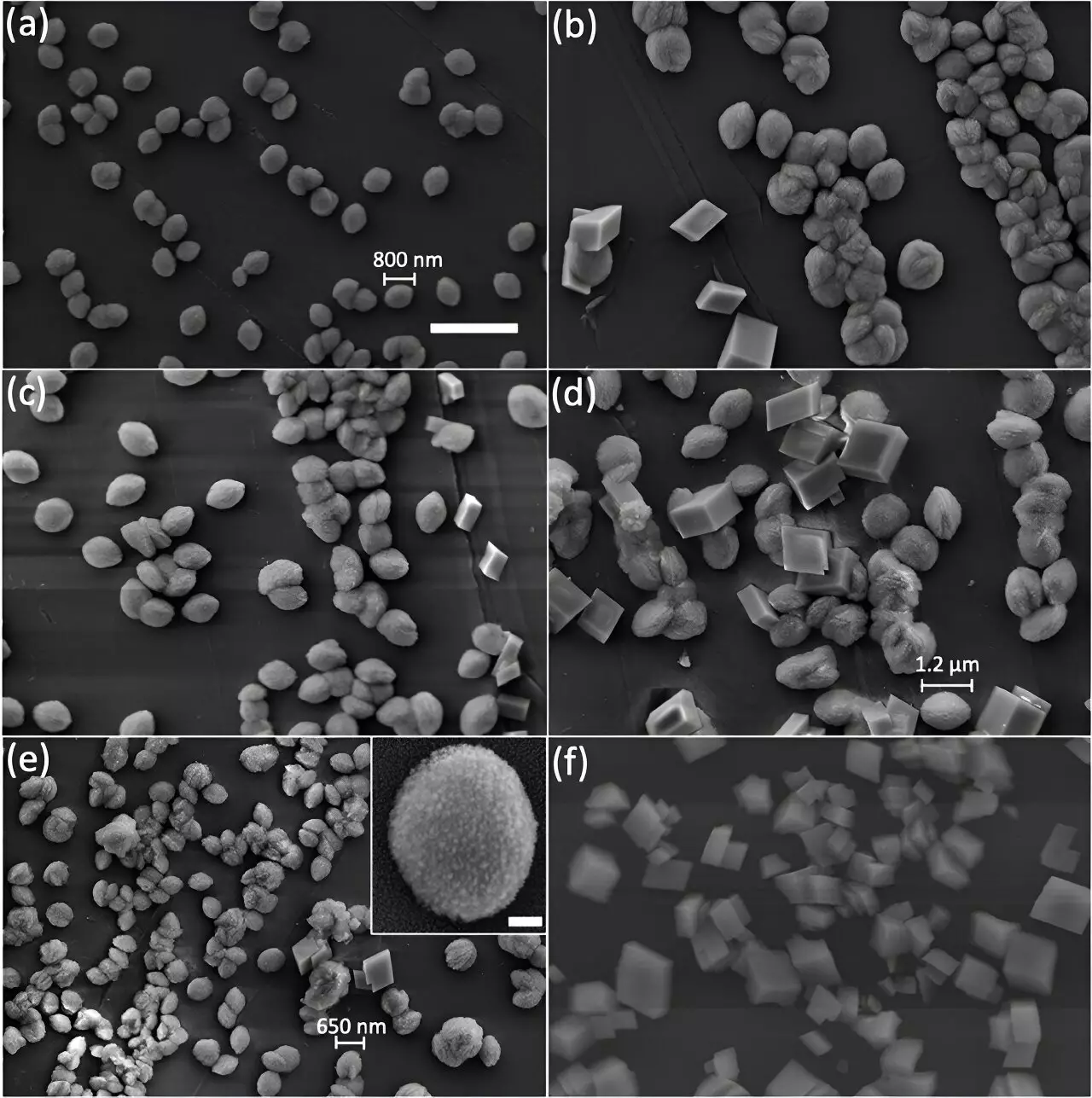
Researchers at Argonne National Laboratory have made significant discoveries regarding the internal structure of calcite particles. By comparing two synthesis approaches using advanced imaging techniques such as scanning electron microscopy (SEM) and Bragg Coherent Diffraction Imaging (BCDI), they found that the way calcite is synthesized can dramatically alter its internal crystallinity and reactivity.
One synthesis approach resulted in the growth of calcite crystals with distorted cube-like shapes, consistent with traditional expectations. However, a different synthesis method led to the formation of calcite crystals composed of ultrasmall nanosized fragments, indicating internal defects. These defects, similar to those found in vaterite, can significantly influence the reactivity and functionality of calcite.
The presence of nanoscopic defects in calcite particles highlights the importance of understanding the internal structure of minerals. Distinguishing between perfect calcite crystals and fragmented ones is crucial, as the latter can have different chemical reactions and properties. Identifying these defects can aid in designing materials with optimized strength, toughness, and enhanced catalytic activities.
The use of advanced imaging techniques like BCDI allows researchers to directly examine the internal structure of calcite particles and determine structure-property relationships. This provides valuable insights into how defects can be utilized to control calcite reactivity and improve the performance of materials in various applications, including catalysis.
The study of calcite and its internal structure is essential for developing a comprehensive understanding of its properties and applications. By unraveling the mechanisms behind calcite synthesis and the impact of internal defects, researchers can pave the way for innovative advancements in material science, environmental protection, and industrial processes. The discoveries made at Argonne National Laboratory shed light on the intricate nature of calcite and highlight the potential for utilizing these insights to create more efficient and sustainable technologies.

Leave a Reply