Plasma Catalysis: A Breakthrough in CO2 Conversion
Researchers at the University of Liverpool have recently made a groundbreaking discovery in the field of sustainable energy production. By utilizing a novel plasma-catalytic process, they have successfully converted carbon dioxide (CO2) into methanol at room temperature and atmospheric pressure, overcoming the limitations of traditional thermal catalysis. This innovative approach offers a more efficient and environmentally friendly method for producing valuable fuels and chemicals.
Plasma Technology: A Game-Changer for Renewable Energy
The use of non-thermal plasma in chemical reactions has proven to be a game-changer in the field of renewable energy. By creating an ionized gas containing energetic electrons and reactive species, plasma can activate strong chemical bonds in inert molecules like CO2, allowing for reactions to occur under mild conditions. This technology offers a flexible and decentralized solution for converting CO2 into methanol, making it a key player in the transition to a sustainable net-zero economy.
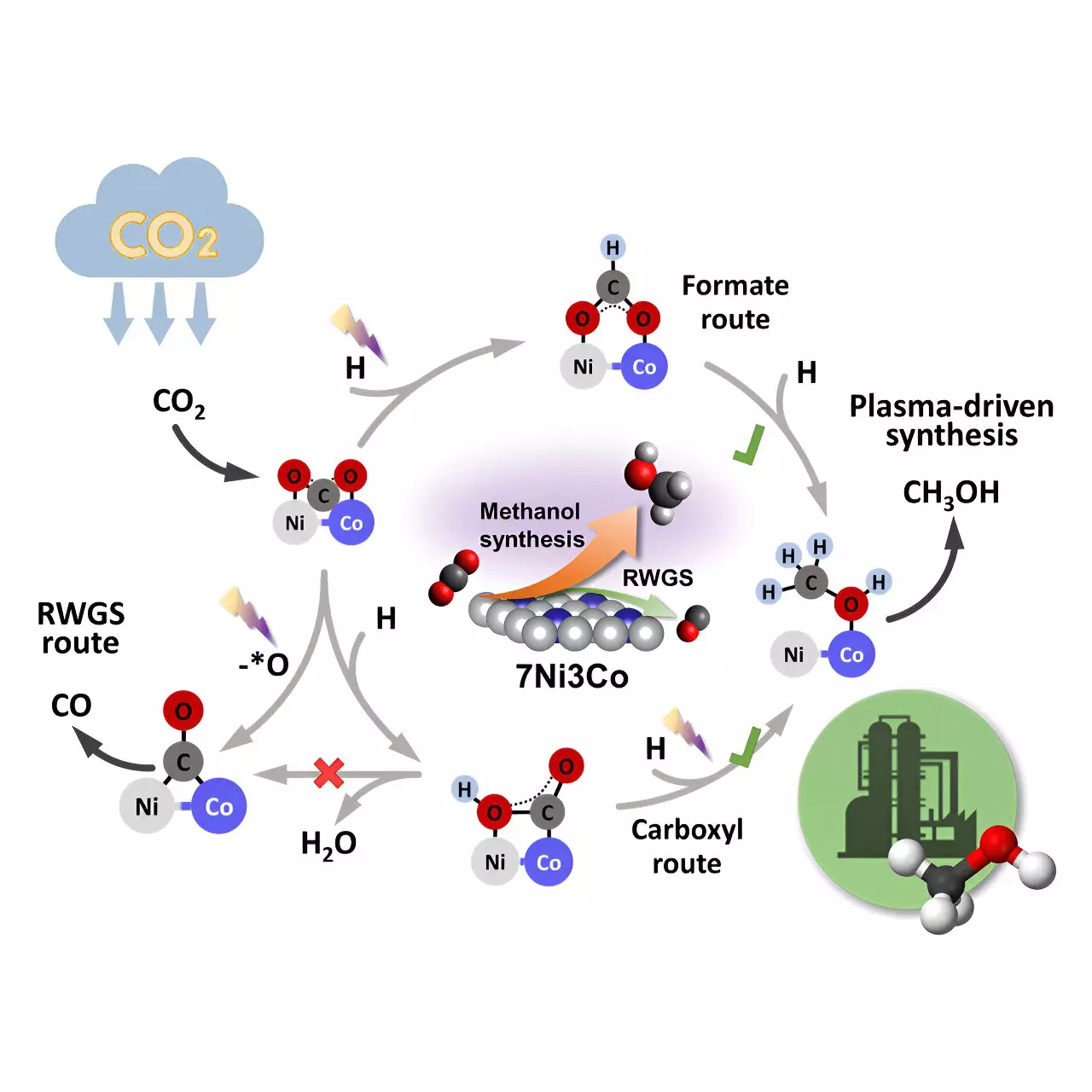
The Role of Bimetallic Catalysts in Methanol Synthesis
One of the key components of this groundbreaking process is the bimetallic Ni-Co catalyst used in the plasma reactor. In situ plasma-coupled Fourier transform infrared (FTIR) characterization and density functional theory (DFT) calculations have revealed that the Ni-Co interface is the primary active center for methanol synthesis. By controlling the distribution of products through asymmetric adsorption of CO2 molecules at the bimetallic interfaces, researchers are able to tailor the weight of each reaction pathway, ultimately increasing the selectivity for methanol production.
The Promise of Plasma Catalysis for Sustainable Fuel Production
Plasma catalysis holds significant promise for the future of sustainable fuel production. By utilizing modular and scalable plasma systems, researchers are able to perform CO2 conversion reactions at ambient conditions, offering a more cost-effective and environmentally friendly alternative to traditional thermal catalytic processes. The ability to power plasma-based systems with intermittent renewable electricity further enhances the feasibility of decentralized fuel and chemical production, making it a key player in the transition to a more sustainable future.
Future Directions and Industrial Applications
The pioneering work done by the University of Liverpool research team opens up promising avenues for future research and industrial applications in the field of catalytic CO2 conversion. By developing plasma processes for CO2 methanation and single-step biogas conversion to methanol, the team is at the forefront of sustainable energy production. With three PCT patents filed in this area, the University of Liverpool is leading the way in plasma catalysis and paving the path towards a more sustainable and environmentally friendly future.
The use of plasma catalysis for the conversion of CO2 into valuable fuels and chemicals represents a significant milestone in the journey towards a sustainable net-zero economy. The groundbreaking work done by the University of Liverpool research team has opened up new possibilities for the future of renewable energy production, offering a more efficient and environmentally friendly alternative to traditional thermal catalysis. By harnessing the power of plasma technology, researchers are paving the way for a more sustainable future for generations to come.

Leave a Reply