A recent study conducted by scientists from China has delved into the intricate process of how short peptide chains come together to form aggregates, shedding light on a crucial aspect of drug stability and material development. Peptides, which are short chains of amino acids, play a fundamental role in the body by constructing structures, catalyzing chemical reactions, and bolstering the immune system. The specific functions of peptides are determined by the interactions between amino acids and how they aggregate to form a three-dimensional structure.
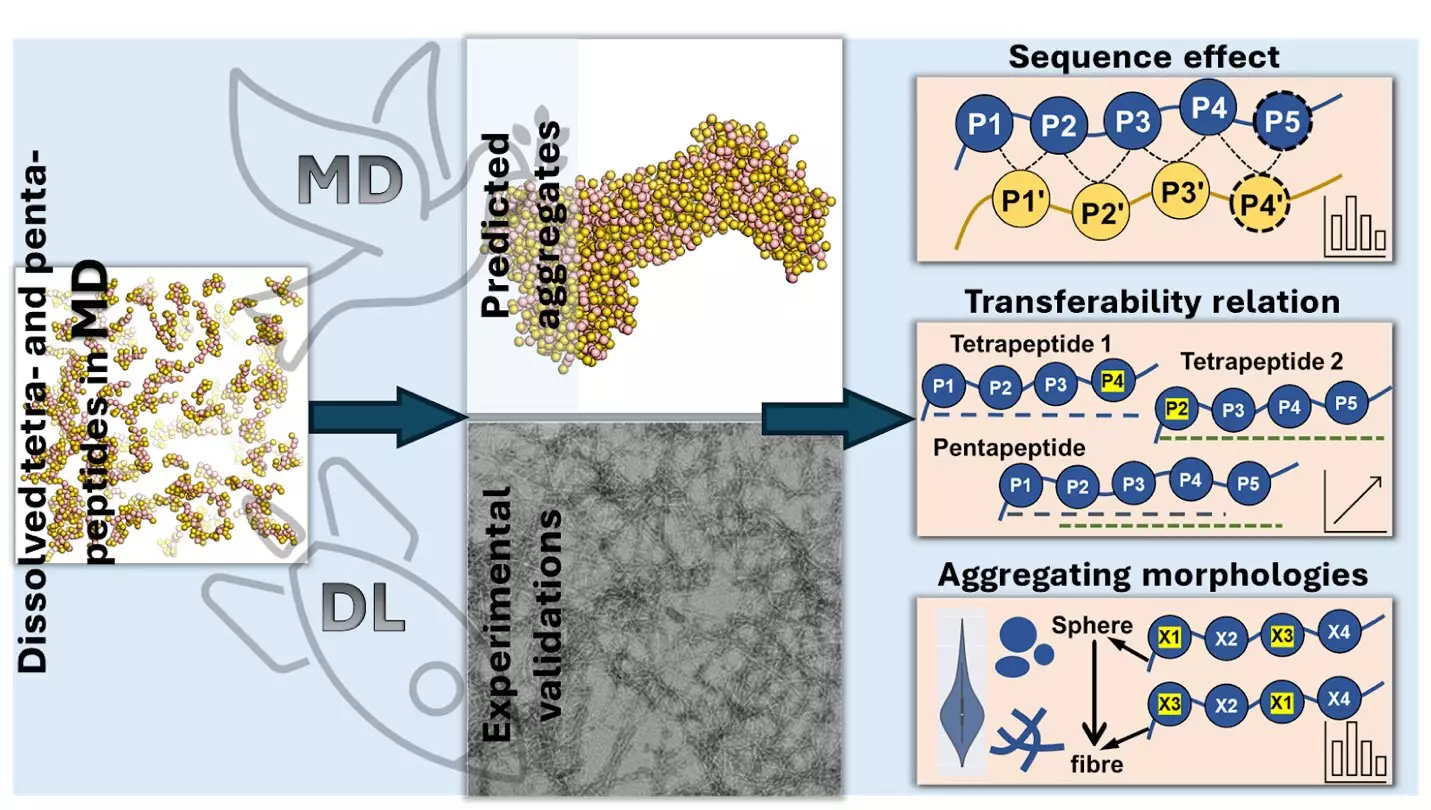
The research team utilized molecular dynamics simulations along with advanced AI techniques to predict the aggregation behavior of tetrapeptides and pentapeptides based on their amino acid sequences. Through the analysis of a vast number of peptide sequences, they discovered that certain amino acids, particularly aromatic ones like tryptophan, phenylalanine, and tyrosine, play a significant role in enhancing aggregation. These aromatic amino acids have ring-shaped structures that attract each other through “π-π” interactions, facilitating the clumping together of peptides. On the other hand, hydrophilic amino acids, such as aspartic acid and glutamic acid, hinder aggregation by strongly interacting with water molecules, preventing peptides from sticking together.
The study further revealed that manipulating the amino acid sequence can have a profound impact on aggregation. For instance, adding aromatic amino acids to the end of a peptide chain promotes aggregation, whereas placing negatively charged amino acids at the beginning diminishes it. Additionally, the types and positions of amino acids determine the shapes in which peptides clump together. Amino acids with a charge tend to form elongated, thread-like structures, while those that repel water tend to create spherical, ball-like clusters.
Understanding the mechanisms of peptide aggregation can have far-reaching implications for various fields, including medicine, material science, and biotechnology. The insights gained from this study can aid in the development of new materials, design of more stable drugs and drug delivery systems, and comprehension of diseases associated with peptide aggregation, such as Alzheimer’s disease. By predicting how peptides will aggregate, researchers can better manage and control their behavior, leading to advancements in biotechnology applications like semiconductors, biosensors, and diagnostics.
Moreover, the integration of advanced AI techniques, such as deep learning models like Transformer Regression Networks, in this study showcases the role of artificial intelligence in scientific discovery. By leveraging AI tools, researchers were able to make accurate predictions about peptide aggregation, paving the way for further advancements in biochemistry, materials science, and computational biology.
The study on peptide aggregation provides valuable insights into the intricate process by which short peptides come together to form aggregates. By unraveling the role of specific amino acids, the influence of amino acid sequences, and the shapes of peptide clusters, researchers have laid the groundwork for advancements in various scientific fields. The integration of AI in this research demonstrates the potential of technology to revolutionize scientific discovery and drive innovation in biochemistry, materials science, and biotechnology.

Leave a Reply