In the quest for a sustainable future, researchers are increasingly turning their attention to carbon dioxide (CO₂) reduction technologies. The conversion of CO₂ into useful chemicals is not only crucial for tackling climate change but also presents an opportunity to innovate in resource management. However, the challenge lies in the efficient conversion of CO₂ into targeted products. Recent studies have unveiled how local chemical environments around copper electrodes can significantly influence this process, suggesting that a meticulous approach to optimization could yield profound benefits.
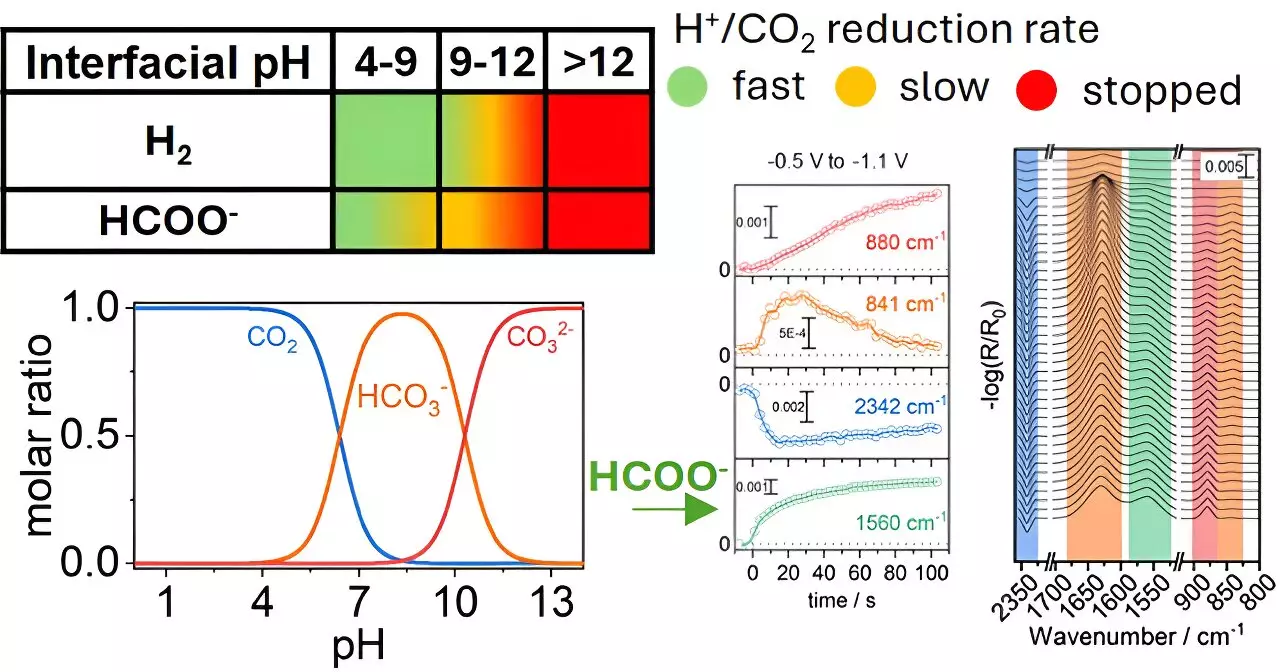
A team from the University of Twente, spearheaded by Georgios Katsoukis, is leading the charge in understanding these dynamics. Their groundbreaking research, published in ACS Catalysis, reveals that adjusting the pH surrounding copper electrodes can drastically alter the efficiency of CO₂ transformation into formate, a compound with broad industrial applications. This insight highlights a pivotal shift in focus from merely examining the electrode materials themselves to a broader perspective encompassing their operational environment.
The study emphasizes that the local chemical environment plays a crucial role in the electrochemical conversion of CO₂. By varying the pH of the aqueous environment at the electrode’s surface, the researchers were able to optimize the interaction between CO₂ and copper. Their findings indicate that it is not sufficient to rely on the catalytic properties of the electrode; the surrounding conditions must also be finely tuned to enhance selectivity towards desired products, such as formate, in CO₂ reduction reactions.
Selectivity in CO₂ reduction reactions has historically presented a considerable hurdle. Different reaction conditions can lead to a plethora of products, complicating efforts to develop efficient conversion technologies. The University of Twente team’s findings challenge the conventional wisdom that emphasizes catalyst composition alone. By demonstrating that nearby chemical conditions can be manipulated to favor certain reactions, the research redirects focus towards a more holistic strategy in catalyst design and system optimization.
Implications for Sustainable Practices
The implications of this research are far-reaching. By controlling the chemical environment around the electrode, scientists may not only enhance the conversion rate but also extend the lifespan of the electrodes themselves. Such advancements could transform CO₂ reduction systems into practical, economically viable solutions for carbon emissions mitigation. This method stands to significantly contribute to a circular economy, where waste products are recycled into valuable resources, ultimately promoting sustainability.
This study from the University of Twente marks a significant step forward in CO₂ reduction technology. By advocating for a dual focus on chemical environments and catalysts, the research establishes a groundwork for future exploration. As scientists continue to optimize these conditions, we move closer to practical applications that can effectively convert CO₂ emissions into useful products, fostering advancements toward a sustainable and resilient economy. The implications of this work encourage ongoing innovation and support the transition towards sustainable industrial processes, a vital endeavor in the fight against climate change.

Leave a Reply