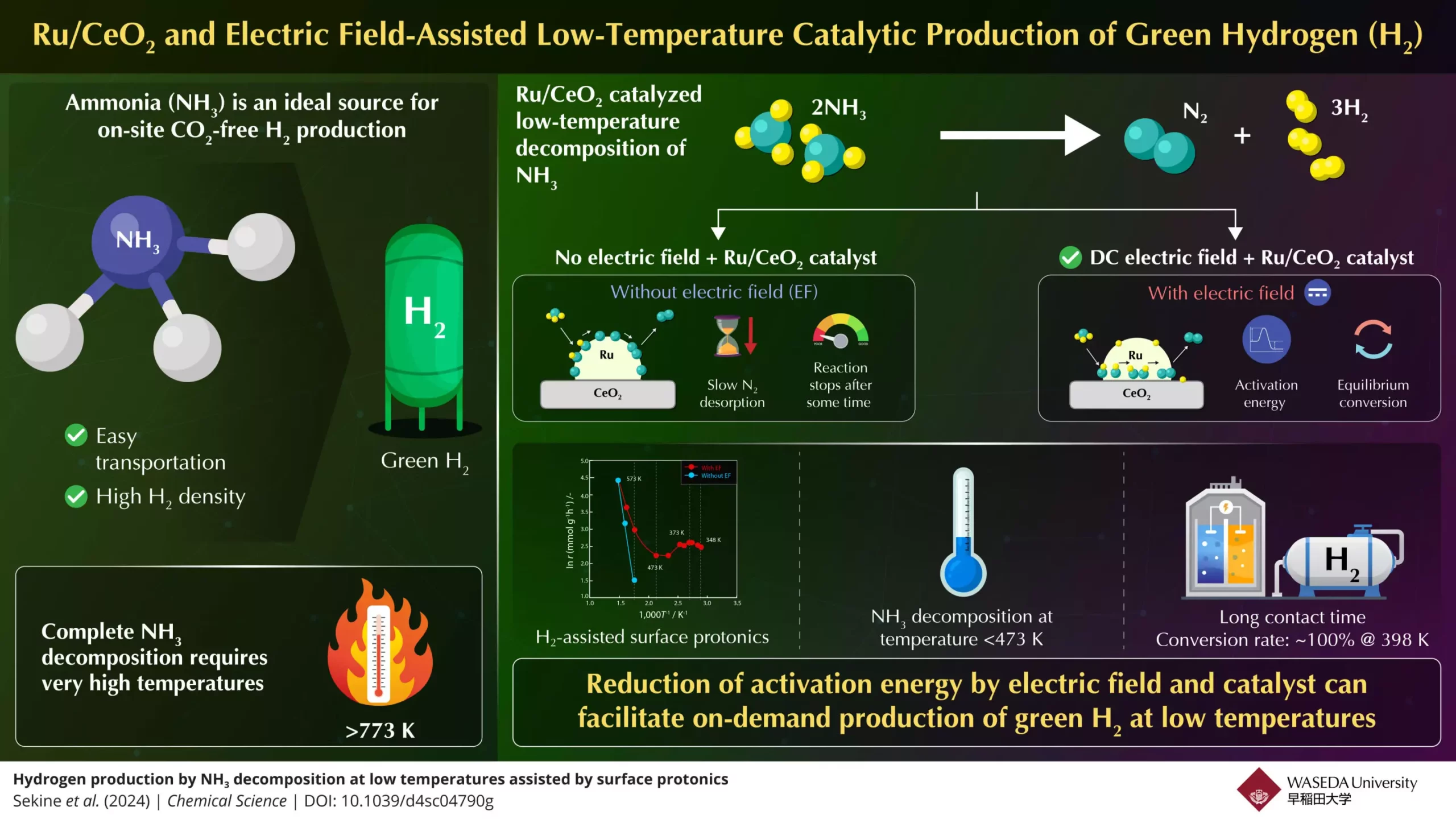
The push towards a sustainable future has thrust hydrogen gas into the limelight, primarily due to its high energy density and its status as a carbon-free fuel source. As the most abundant element in the universe, hydrogen holds immense potential, yet it is typically found in bound forms within various chemical compounds, including ammonia. For various reasons, ammonia is deemed a highly viable hydrogen carrier. Its ready availability, substantial hydrogen content—comprising approximately 17.6% of its mass—and ease of liquefaction and transportation present a strong case for its utilization in energy systems. However, despite these advantages, the practical application of ammonia as a green hydrogen source faces significant challenges, particularly its requirement for extremely high temperatures to enable the decomposition reaction necessary for hydrogen production.
The decomposition of ammonia (NH3) into hydrogen (H2) is typically a precarious process requiring temperatures exceeding 773K, which not only presents logistical hurdles but also raises energy costs associated with heating. For fuel cells and internal combustion engines, high conversion rates at lower temperatures are essential to promote efficiency and sustainability. This creates a paradox where the desirable features of ammonia as a hydrogen carrier are offset by the energy-intensive processes required for its utilization. The quest for an efficient means of generating hydrogen on-demand from ammonia, therefore, represents a critical need in the transition to alternative fuel sources.
In light of these challenges, recent advancements from Waseda University showcase promising innovations that could change the landscape of hydrogen production. Led by Professor Yasushi Sekine and his team—including Yukino Ofuchi, Sae Doi, and Kenta Mitarai—research has demonstrated a novel approach that facilitates the ammonia-to-hydrogen conversion process at significantly lower temperatures.
What differentiates this innovative process is the application of an electric field in conjunction with a highly active catalyst, Zinc Oxide–Ruthenium (Ru/CeO2). By utilizing this combination, the researchers were able to attain high ammonia conversion rates even at temperatures below 473K—a striking achievement in the field. The team’s experimental setup revealed that when subjected to an electric field, proton conduction at the catalyst’s surface improved notably, leading to a decrease in the activation energy required for reactions and facilitating rapid decomposition of ammonia into hydrogen.
Further technical investigations revealed the primary rate-limiting factor in ammonia decomposition is the nitrogen desorption reaction at lower temperatures. The introduction of the electric field effectively mitigates this issue by enhancing surface protonics, a process where protons hop across the catalyst’s surface, thereby optimizing reaction pathways. This is important, as traditional methods often fail after a short duration due to nitrogen desorption limitations, stalling the ammonia decomposition process.
In a crucial part of their experiment, the team noted that with adequate contact time, they were able to achieve a remarkable 100% conversion rate at 398K—well below the typical equilibrium demands of the reaction. This groundbreaking finding is set against the backdrop of ongoing concerns regarding the efficiency of hydrogen production methods. The ability to surpass equilibrium conversion rates means that this process could dramatically shift current paradigms in hydrogen utilization.
The implications of this research extend beyond theoretical prospects; they suggest that clean hydrogen fuels could become readily available for a variety of applications, potentially revolutionizing the energy landscape. As a result of their findings, the researchers advocate for the widespread adoption of their method, arguing that it paves the way for clean alternative fuels by simplifying the process of synthesizing CO2-free hydrogen.
While the journey towards a sustainable hydrogen future is fraught with challenges, innovative techniques such as electric field-assisted catalysis represent hopeful strides towards achieving practical, efficient solutions. Innovations like these not only underscore the necessity of flexible and adaptive methods in energy production but also highlight the enormous potential inherent in ammonia as a hydrogen carrier. The findings from Waseda University illuminate a path forward in the relentless pursuit of clean energy alternatives.

Leave a Reply