Recent findings from Rice University, spearheaded by Jason Hafner and his research team, have illuminated the intricate role of cholesterol within cell membranes. Published in the Journal of Physical Chemistry, this study provides insights that could significantly impact our understanding of diseases where membrane organization plays a crucial role, particularly cancer. Cholesterol has long been recognized as a vital molecule in the formation and regulation of biomembranes, which primarily consist of proteins and lipids. However, the specific mechanisms by which cholesterol affects membrane structure and receptor functions have remained elusive.
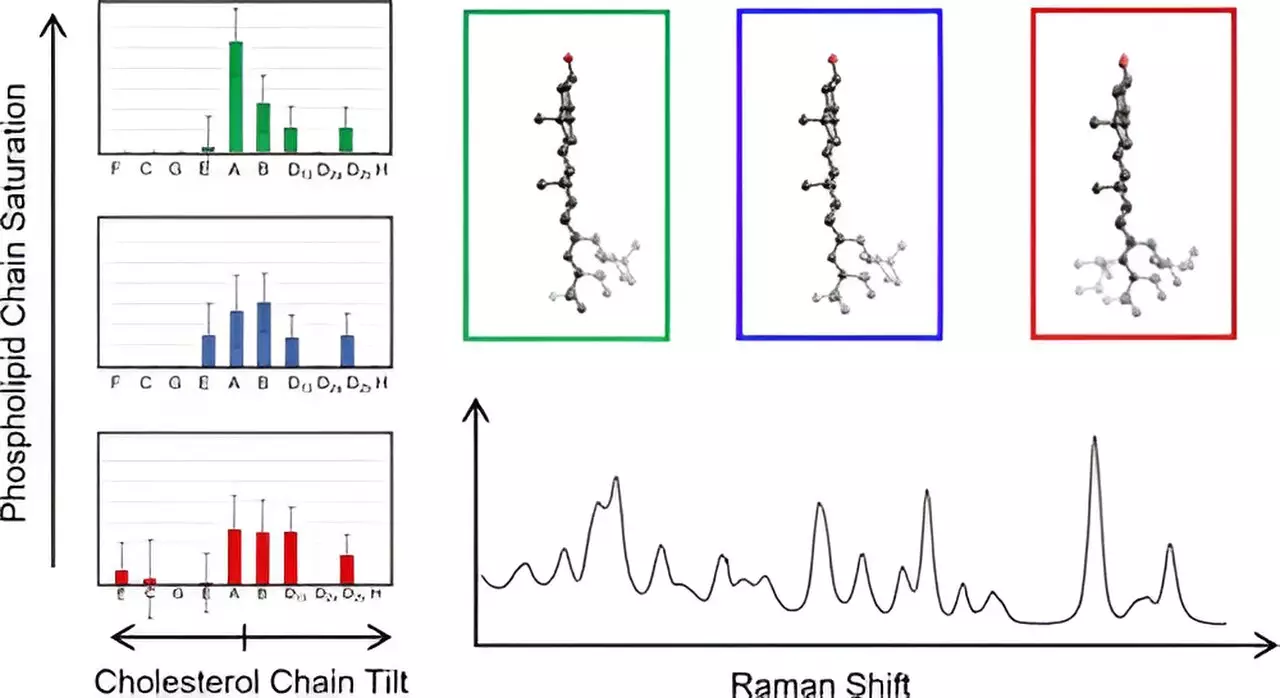
To dissect the complexities surrounding cholesterol’s interactions with cell membranes, Hafner’s lab employed Raman spectroscopy, a cutting-edge technique that employs laser light to analyze molecular vibrations. This method yields in-depth vibrational spectra that reveal essential molecular information. The research concentrated on the behavior of cholesterol molecules embedded within lipid membranes, contrasting the empirical results obtained through spectroscopy with calculations derived from density functional theory—a computational method grounded in quantum mechanics. Such innovative approaches allowed researchers to probe the distinctive vibrational characteristics of cholesterol, bridging theoretical calculations with real-world molecular behavior.
A remarkable focus of the study was on the unique fused ring structure of cholesterol and its characteristic eight-carbon chain. The research team generated Raman spectra for 60 cholesterol variants, uncovering a novel classification system based on variations in the carbon chain’s orientation relative to the ring structure. This revelation opens doors to understanding the subtle structural variations that could impact cholesterol’s functionality in membranes. Most notably, the study marked the first instance of researchers directly measuring cholesterol chain structures in their natural environment, paving the way for future explorations in membrane biochemistry.
The implications of this research extend beyond mere biochemical curiosity. According to Hafner, a professor of both physics and chemistry, the understanding gained from this study could lay foundational knowledge for further inquiries into diseases linked to cell membrane dysfunction. Specifically, in cancer research, where abnormalities in membrane organization are frequently observed, this study offers potential new pathways for therapeutic investigations. Understanding how cholesterol organizes within the membrane environment could lead to novel approaches in targeting the underlying mechanisms of cancer and related diseases.
The collaborative efforts of Hafner and his team, including graduate and undergraduate researchers, have propelled forward the understanding of an integral component of cell membranes—cholesterol. Their breakthrough serves not only as a rich source of data for academia but also as a potential catalyst for advancements in medical research. As studies evolve, the knowledge gleaned from investigating the nuances of membrane organization will undoubtedly pave the way for significant strides in our understanding of cellular dysfunction and disease. The prospect of harnessing this information for practical applications remains an exciting frontier for both scientists and medical professionals alike.

Leave a Reply