Recent research has cast a spotlight on high entropy oxides, a category of materials that have gained traction due to their diverse electrochemical properties and potential applications in modern electronics. These materials are distinguished by their complex composition, typically involving multiple transition metal oxides combined in varying ratios. As scientists delve deeper into understanding these materials, it becomes evident that not only composition but also the synthesis method plays a crucial role in determining the structural and functional properties of high entropy oxides.
Significance of Synthesis Techniques
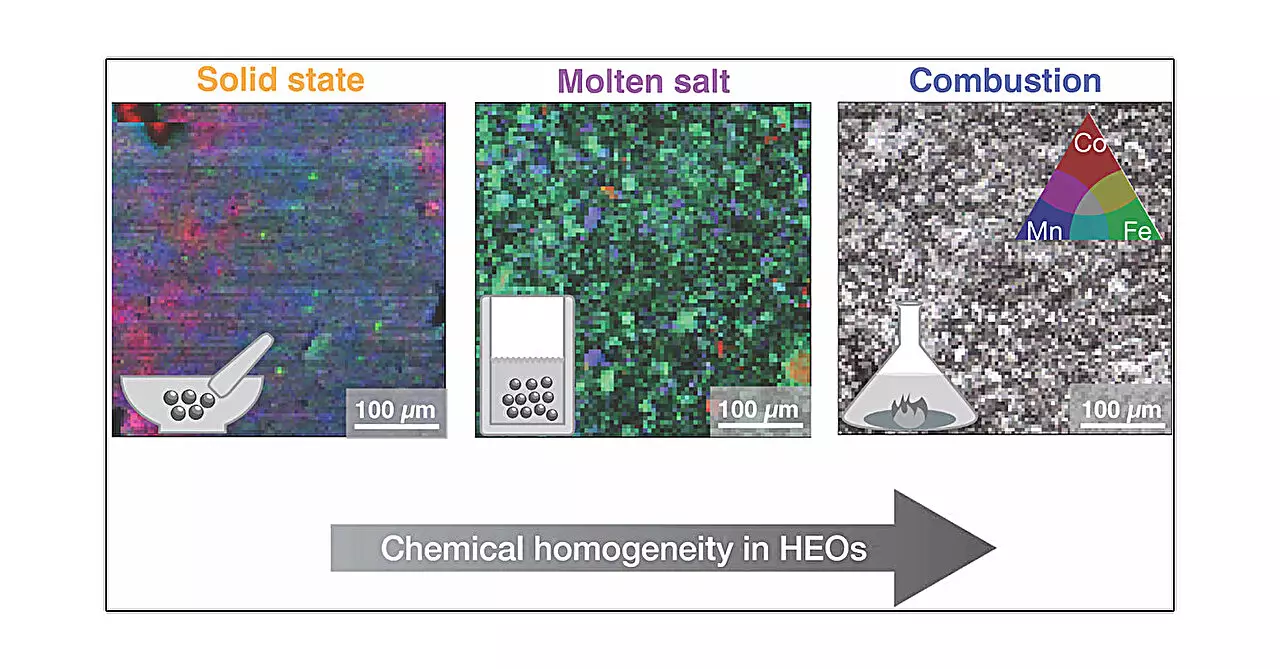
The synthesis of high entropy oxides can be likened to art; the method chosen can profoundly affect the final outcome, akin to an artist selecting different brushes or palettes. A recent study published in the Journal of the American Chemical Society illustrates this concept through the examination of five distinct synthesis methods: solid-state synthesis, high-pressure synthesis, hydrothermal synthesis, molten salt synthesis, and combustion synthesis. Each method incorporates unique thermal, pressure, and chemical variables that significantly influence the characteristics of the resulting materials.
The research, spearheaded by Alannah Hallas at the University of British Columbia, highlights how these variations in synthesis methods lead to different local structures within the material, despite an unchanged average structure. Such findings underscore the importance of selecting appropriate synthesis techniques based on the intended application of the material.
The solid-state synthesis method, perhaps the most straightforward approach, involves blending metal oxides and heating them, akin to baking a cake. This method has been widely used for its simplicity and efficacy. However, the study showed that this method yielded materials with less homogeneity compared to others.
In contrast, the high-pressure synthesis method applies external pressure during the heating phase, resulting in altered material formation dynamics. Pressure can facilitate more compact structures and induce unique electronic properties that could be advantageous in specific applications.
Hydrothermal synthesis, a technique that mirrors natural mineral formation, employs high temperatures and pressures in a water-based environment to promote crystal growth. This method has garnered attention for its potential to create highly ordered structures that might enhance the electrochemical performance of the resulting materials.
Another fascinating method is molten salt synthesis, where melted metal salts serve as a medium. As this viscous substance cools, it allows for the controlled precipitation of crystals, promoting uniform sizing and potentially more predictable electrical properties.
Lastly, combustion synthesis involves a rapid exothermic reaction that transforms metal salt gels into the desired oxide. This method is especially noteworthy for its ability to produce homogeneous materials quickly, appealing for large-scale industrial applications.
The implications of these findings are significant, particularly regarding energy applications. High entropy oxides exhibit promise for various energy challenges, including battery technology and catalysis. The study’s lead author, Mario Ulises González-Rivas, emphasizes that the diverse structural variations induced by different synthesis methods can have far-reaching effects on the implementation of these materials in energy systems. By offering a new optimization axis, this research provides essential insights for future developments, encouraging further exploration into the interaction between synthesis methods and material properties.
A Collaborative Effort in Material Science
Collaboration has been a cornerstone of this study, bringing together researchers from multiple institutions, including the University of Saskatchewan and the Max Planck Institute. This interdisciplinary approach not only broadens the expertise applied to the research but also enhances the potential for innovative discoveries in the field of materials science.
As researchers deepen their understanding of high entropy oxides, the meticulous selection of synthesis methods emerges as a pivotal factor in optimizing both structural and functional properties. This study not only opens new avenues for research but also lays a foundation for harnessing the full potential of high entropy oxides in addressing pressing energy challenges of our time. The future of this research promises to be dynamic, offering the possibility of unprecedented advances in material applications across a spectrum of technologies.

Leave a Reply