Have you ever experienced the frustration of assembling a piece of furniture from IKEA, only to wish that the components could magically join together? This concept of effortless assembly is paralleled in the realm of biology, where self-assembly plays a critical role in the formation of various complex structures. From proteins and cell membranes to whole viruses, the ability of components to autonomously come together is foundational in nature. The discipline of supramolecular chemistry seeks to emulate this phenomenon by constructing large molecular complexes from smaller, predetermined units.
Supramolecular chemistry involves manipulating the attractive forces between different polymer chains to create “smart materials.” These materials have the ability to respond dynamically to external stimuli, such as chemical changes in their environment. However, despite the potential applications of this field, the intricacies of supramolecular interactions are still not completely understood.
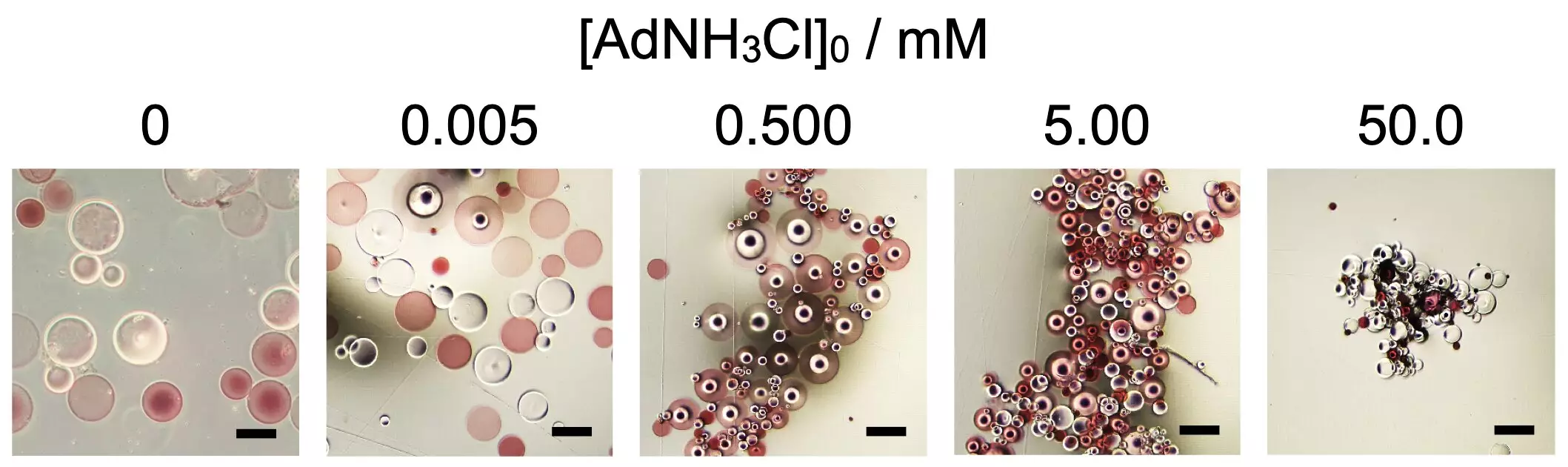
A recent study from Osaka University has shed light on these mechanisms, demonstrating how specific additives can enhance the self-assembly process of microparticles made from poly(sodium acrylate), a highly absorbent polymer. By functionalizing the polymer chains with β-cyclodextrin and adamantane, researchers were able to create systems that only began to assemble at a critical concentration of 1-adamantanamine hydrochloride.
One of the most captivating aspects of this research is the inspiration drawn from biological proteins. Proteins are essentially long chains of amino acids that fold into intricate shapes depending on various forces at play, such as hydrogen bonds and electrostatic interactions. A similar principle applies to the microparticles in the Osaka study. The concentration of the additive influenced the resulting shape of the assemblies, ranging from more spherical structures to elongated forms. This discovery not only demonstrates the power of supramolecular chemistry but also parallels the mechanisms of shape control found in living organisms.
Researchers Akihito Hashidzume and Akira Harada assert that understanding these fundamental principles could provide insights into the morphological diversity seen in nature. By controlling the parameters of assembly, scientists could potentially reveal the foundational rules behind the shapes of different biological entities.
The implications of this research stretch far beyond theoretical understanding. By harnessing the principles of self-assembly, future innovations could lead to the development of novel materials that shift and change in response to their surroundings. Such smart materials could have diverse applications in fields ranging from medicine to materials science.
The exploration of supramolecular chemistry and self-assembly not only offers a glimpse into the fundamental workings of biological systems but also paves the way for transformative materials capable of intricate manipulation. As researchers continue to decode these interactions, the potential for groundbreaking advancements becomes increasingly tangible. The art of self-assembly is not merely a biological curiosity; it represents a frontier for innovation and creativity in material science.

Leave a Reply