In the hunt for sustainable energy solutions, hydrogen energy holds immense potential. As a clean and renewable source, hydrogen is gaining traction in various sectors, promising a future characterized by low-carbon emissions and abundant energy. The increasing demand for efficient hydrogen production methods highlights the importance of innovative technologies that can facilitate the transition to a sustainable energy economy. Among these technologies, the process of electrochemical water splitting has emerged as a focal point, generating interest due to its promising capabilities in hydrogen generation. However, one of the significant hurdles in optimizing this process lies in the oxygen evolution reaction (OER), which is essential for ensuring a high degree of energy conversion efficiency.
The OER, while crucial for water decomposition, faces inherent kinetic limitations, particularly at the anode. These limitations can drastically reduce the overall efficiency of the electrochemical process, underscoring the critical need for effective catalysts that enhance OER performance. Traditional catalysts have made strides in improving efficiency, but ongoing research seeks new, more potent materials to push the boundaries of hydrogen production further. This is where the role of single-atom catalysts (SACs) becomes transformative, offering a promising pathway to overcome current limitations associated with catalytic efficacy.
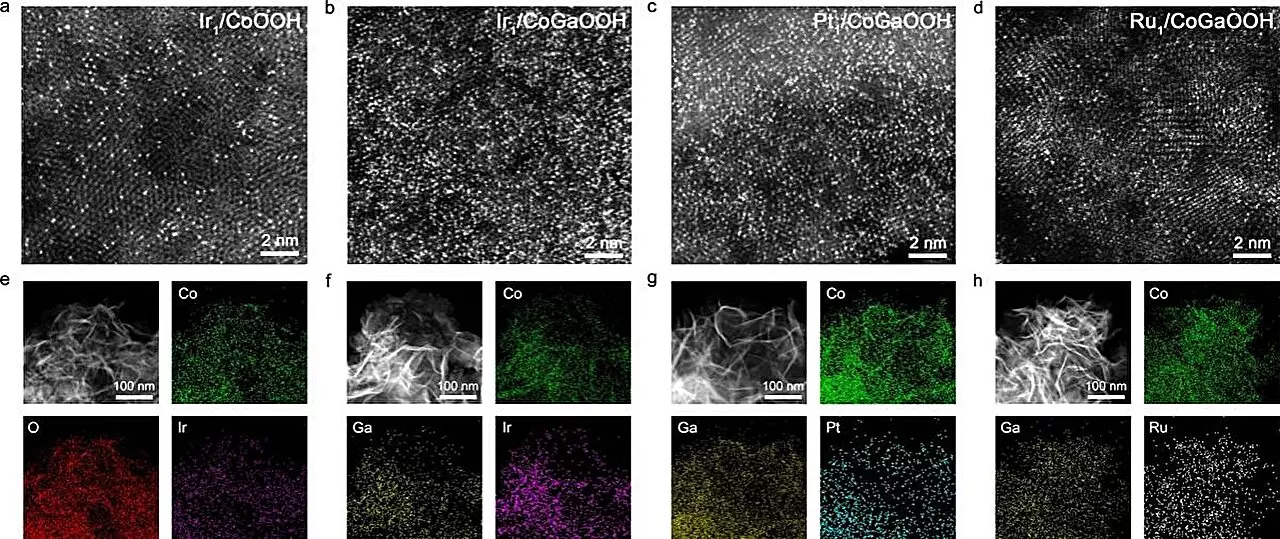
Recent advancements in the field have been spearheaded by a team of researchers led by Prof. Bao Jun at the University of Science and Technology of China (USTC). Their innovative exploration of high-density single-atom catalysts has illuminated new pathways of synergy, wherein the spatial proximity of individual catalyst atoms significantly influences catalytic performance. Specifically, they have developed a cobalt-based, oxide-supported Ir single-atom catalyst, framed within a network conducive to enhanced OER activity. Their findings, published in Angewandte Chemie, demonstrate that optimizing the spatial arrangement of atoms—and thereby leveraging neighboring synergetic interactions—can lead to profound improvements in catalyst efficiency.
The research emphasizes that the arrangement and density of single atoms on a catalyst’s surface directly contribute to its performance. As the distance between individual atoms decreases, unique synergy emerges that can optimize how the catalyst interacts with reaction intermediates. The USTC team innovated a technique involving the introduction of gallium (Ga) atoms into a cobalt-based oxide matrix, leading to significant improvements in the electronic structure of the interface where the single atoms anchor. This manipulation not only increased the stability of the Ir atoms within this structure but also fostered catalytic environments tailored for electrochemical reduction.
During experimentation, the team recorded striking performance metrics, identifying an overpotential of just 170 mV at a current density of 10 mA cm-2 for their high-density Ir single-atom catalyst, referred to as Nei-Ir1/CoGaOOH. The catalyst exhibited remarkable stability for over 2000 hours, showcasing its viability for prolonged operational use—a critical characteristic for practical applications. Moreover, it sustained an operational current density of 1 A cm-2 in alkaline environments for more than 50 hours. These results underscore significant advancements in stability and efficiency for OER catalysts.
In situ studies further elucidated the performance dynamics of the high-density Ir catalyst. The researchers found that the catalyst’s efficiency was less about the electronic reconfiguration of active sites and more a function of the synergistic interactions among neighboring single atoms. Notably, these interactions stabilized the *OOH intermediates, a crucial component in the OER process, via hydrogen bonding. This stabilization effectively lowered the energy barrier associated with the reaction, resulting in enhanced catalytic performance—a crucial insight for future catalyst design.
The groundbreaking research on high-density single-atom catalysts poised to revolutionize hydrogen production brings to light the intricate interplay between atomic structure and catalytic efficacy. By uncovering the mechanisms behind these advancements, researchers are poised to further refine electrochemical OER catalysts, guiding the development of efficient, sustainable hydrogen energy technologies. As we seek to mitigate the current energy crisis and transition toward lower-carbon alternatives, the quest for innovative catalysts like those developed at USTC represents a significant leap forward in harnessing hydrogen’s potential as a clean energy source. The future of hydrogen energy is not just promising; it is rapidly evolving, marked by scientific discoveries that hold the key to a sustainable energy landscape.

Leave a Reply