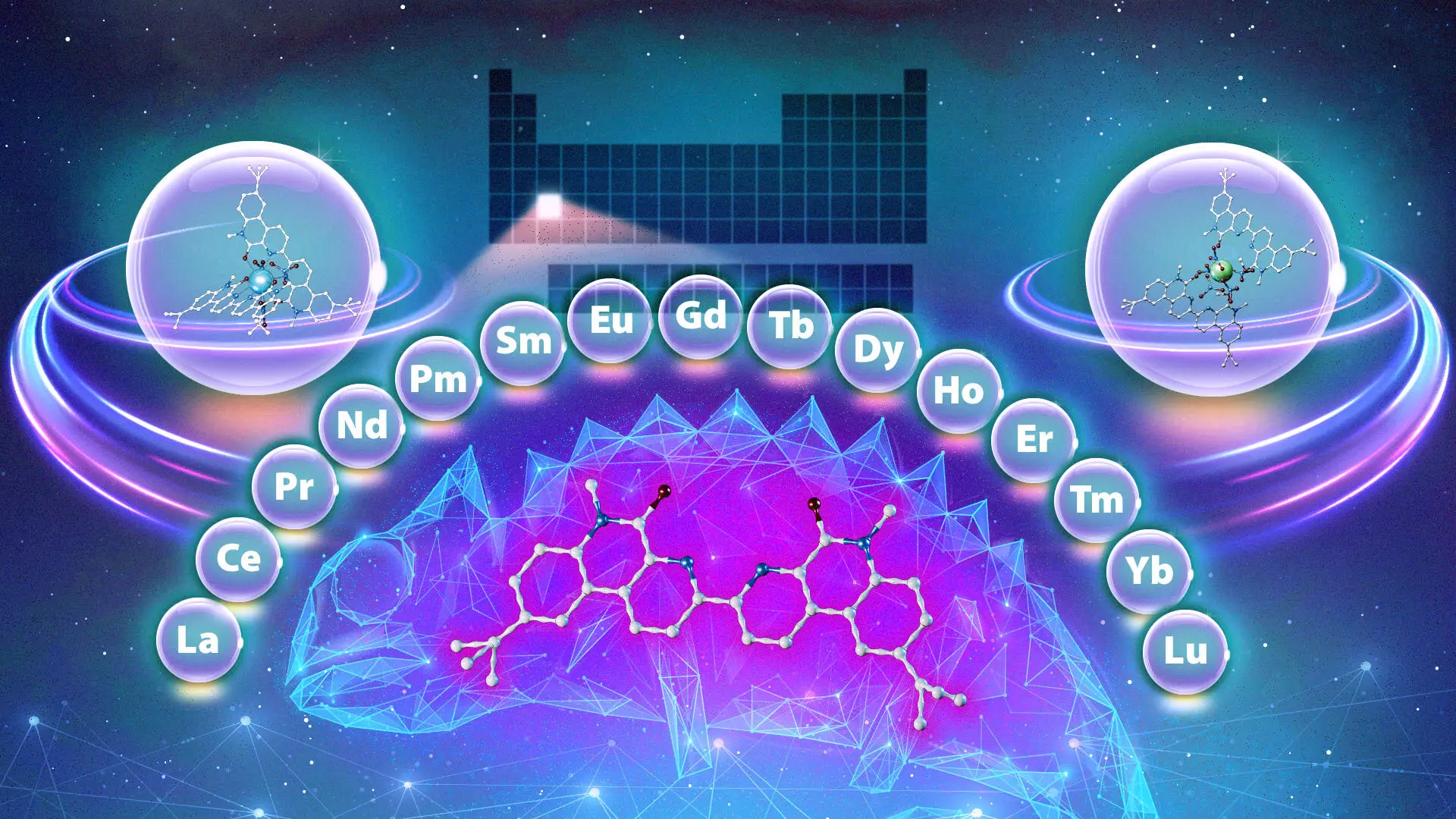
The quest for cleaner energy and innovative technologies has shown us the undeniable importance of rare-earth metals, particularly the lanthanides, which play a crucial role in various applications ranging from medical imaging to electronics. The breakthrough research conducted at Oak Ridge National Laboratory (ORNL), in collaboration with Vanderbilt University, has unveiled a “chemical chameleon,” a ligand that can transform the challenging, costly, and environmentally damaging process of purifying these essential metals.
Rare-earth metals, contrary to their name, are not exceptionally rare. Lanthanides, a set of 15 elements, are found abundantly in nature, often alongside more familiar metals like copper and lead. Their use in cutting-edge technologies is vast, but the intrinsic properties that make them so valuable are only activated when they are isolated from other elements. The challenge lies in the difficulty in separating these metals, as they share very similar sizes and chemical properties. The process necessitates precision, making it both time-consuming and resource-intensive.
The extraction and purification of lanthanides involve complex chemical processes known as separation science. Researchers employ ligands—molecules that can selectively bind to specific metals and assist in their extraction. While effective, traditional methods often require a laborious system of steps that lead to excessive waste. Hence, the need for innovation in this domain has never been more pressing.
The discovery of the chemical chameleon is an exciting step towards overcoming the hurdles faced in rare-earth metal purification. This unique ligand exhibits the ability to adapt its binding preferences based on the composition of the surrounding environment, similar to how a chameleon changes its color. The research, published in the Journal of the American Chemical Society, posits that this ligand can selectively bind to different lanthanides according to the acidity of the solution and the duration of interaction.
Santa Jansone-Popova, a lead researcher from ORNL, highlighted this extraordinary ability by explaining that traditional ligands are typically biased toward either lighter or heavier lanthanides. However, the chameleon ligand’s versatility allows it to bind to a broader spectrum of lanthanides in any order—an unprecedented advancement that could revolutionize the methodology of separation processes.
The ability to use a single compound to carry out multiple separations offers profound implications for the efficiency of industrial practices. By streamlining the process, researchers anticipate that this method not only reduces the number of stages involved but also mitigates waste production, fostering a more sustainable approach to rare-earth metal extraction. The environmental implications could be significant as industries seek to lessen their ecological footprint amidst growing concerns over resource depletion and environmental degradation.
The traditional practices for separation are fraught with drawbacks—both in terms of costs and environmental consequences. The chameleon ligand has the potential to address these issues directly. By effectively diminishing the time and expense associated with current methods, this groundbreaking research aligns with global movements toward cleaner and more sustainable technological solutions.
This discovery opens new avenues for future research, as the behavior of the chameleon ligand emphasizes that there is still much to understand about the structural and functional diversity among ligands. The researchers believe that now, with a clearer understanding of how certain structures exhibit unique behavior, there is potential to design and discover an array of new compounds with similar capabilities.
As Ilja Popovs stated, the study highlights the intricate relationship between a ligand’s structure and its functionality, suggesting that the field of separation science may soon witness innovations that could substantially widen its applications across industries.
Overall, this research marks not just a significant advancement in chemical sciences but also poses a broader reflection on how scientific exploration can yield revolutionary changes in technology and environmental sustainability. With the stakes as high as they are in our quest for renewable energy resources, the discovery of the chemical chameleon could indeed be a pivotal step in this endeavor.

Leave a Reply