Hydrogen fuel has long been heralded as a potential game-changer in the realm of clean energy. However, despite its promise, significant hurdles remain in the way of its widespread adoption. The inherent characteristics of hydrogen, particularly its storage requirements, present major challenges. Hydrogen occupies a considerable volume compared to traditional fuels like gasoline, complicating storage solutions. Consequently, researchers worldwide are intensely focused on overcoming these challenges to harness hydrogen’s potential as a viable power source.
Recent collaborations among chemists from prominent institutions—University of Hong Kong, Northwestern University, and Duke University—have led to a noteworthy advancement in hydrogen storage technology. The culmination of their efforts is a novel supramolecular material that successfully compresses hydrogen while alleviating the issue of excessive weight. This breakthrough was reported in “Nature Chemistry,” showcasing the potential of porous organic crystals as a solution to hydrogen storage limitations.
The innovation is significant in that it addresses specific criteria mandated by the U.S. Department of Energy concerning hydrogen storage. The first criterion demands a minimum hydrogen storage capacity of 50 grams per liter of the storage medium. The second involves achieving a favorable weight ratio, where hydrogen must comprise at least 6.5% of the total weight of the storage solution. Historically, various materials have failed to meet these stringent targets, but this new development signifies a promising step forward.
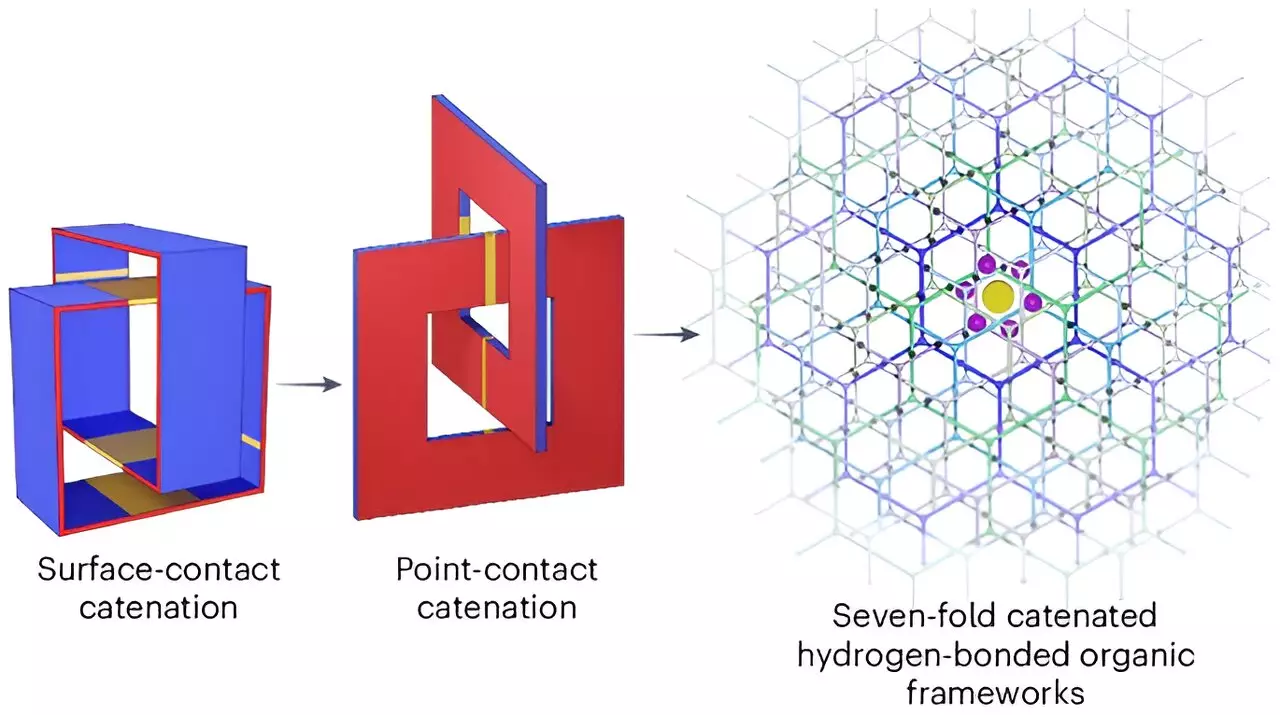
The success of the material lies in its unique construction. The team has synthesized a complex network of organic molecules that form robust crystals arranged in a honeycomb structure. This design features pores specifically sized to accommodate hydrogen molecules, allowing them to bond with the crystal framework. It is noteworthy that this interlinked structure not only facilitates efficient and stable hydrogen retention but also addresses the porousness limitations seen in previous attempts by different researchers. Laboratory tests revealed that this newly formed material can store an impressive 53.7 grams of hydrogen per liter while also ensuring that hydrogen constitutes approximately 9.3% of the overall weight—both metrics exceeding the set benchmarks.
While these findings are undoubtedly encouraging, the solution is not without its drawbacks. One significant limitation of the new hydrogen storage method is its reliance on cryogenic cooling systems. In practical terms, this requirement could render the system bulky and potentially cost-prohibitive for commercial applications. Therefore, while researchers celebrate this scientific triumph, practical implementations may still need to resolve issues of infrastructure, efficiency, and cost-effectiveness.
While the development of this new supramolecular material signals a promising advancement in the field of hydrogen storage, it is vital to address the challenges associated with its application in real-world scenarios. The collaboration of leading academic institutions exemplifies the importance of teamwork in scientific innovation, which could eventually lead to groundbreaking advancements in clean energy technology. The future of hydrogen as a clean energy source may hinge on overcoming these early obstacles, making ongoing research and development paramount.

Leave a Reply