Photocatalysis, the harnessing of light to accelerate chemical reactions, has garnered significant attention in recent years for its potential applications in energy conversion, pollution control, and organic synthesis. This technology draws inspiration from natural processes, such as photosynthesis, where light energy is transformed into chemical energy in plants. Typically, photocatalytic reactions require high temperatures and harsh conditions, making the ability to utilize light for such transformations particularly advantageous. However, for photocatalysis to be widely adopted in practical applications, an enhancement of quantum efficiency is essential. Thus, research has continuously focused on developing photocatalysts that exhibit improved efficiency, with molecular dyads emerging as a prominent area of study.
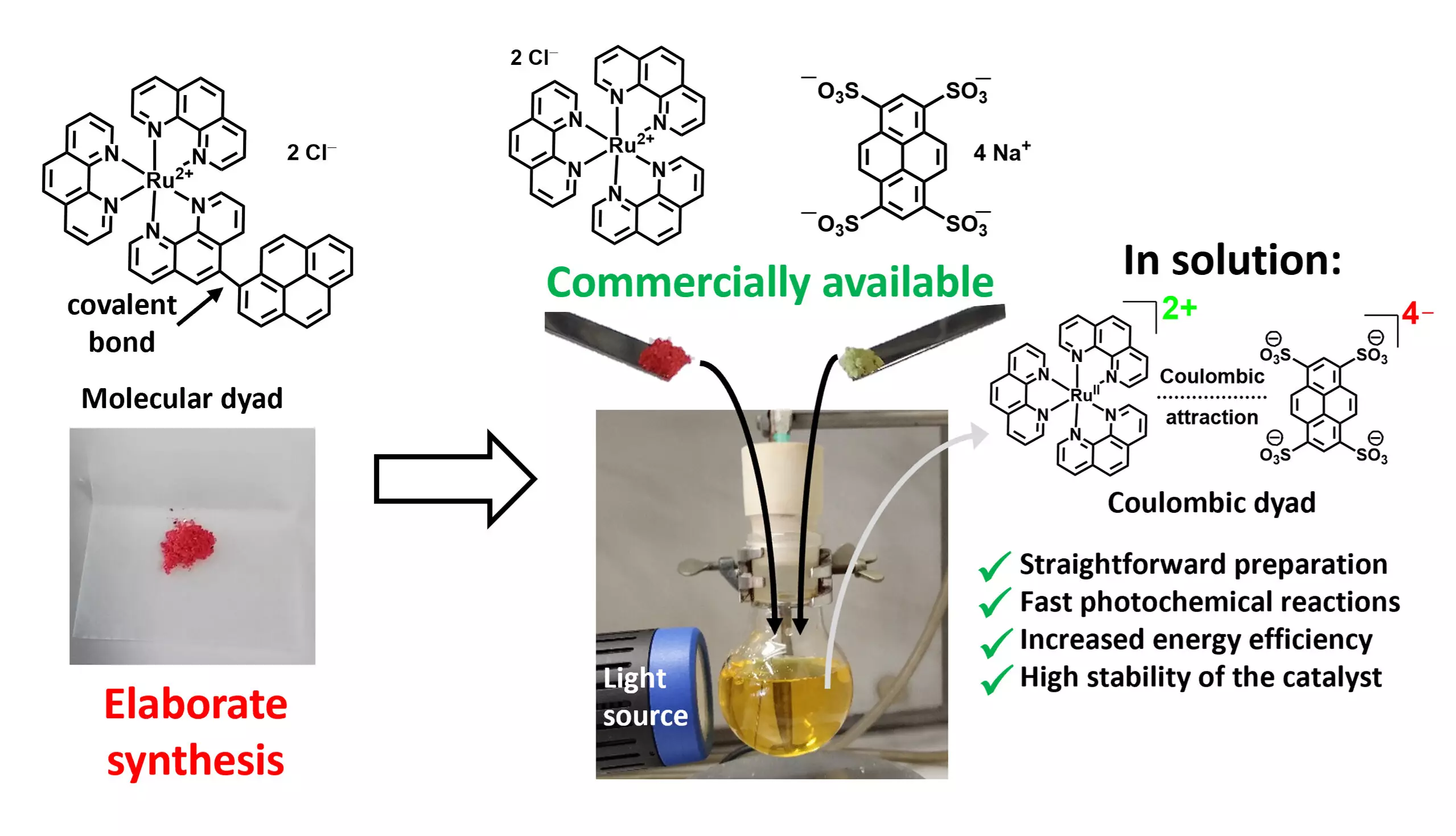
Molecular dyads, which consist of two interconnected photoactive components, have demonstrated remarkable photocatalytic efficiencies. Nonetheless, their multistep synthesis often makes them too costly for large-scale utilization. Researchers around the globe are striving to turn less expensive, Earth-abundant metals into effective photocatalysts, hoping to replicate the efficiency of their more costly counterparts like iridium, ruthenium, and osmium. The complexity and resource demands associated with synthesizing sophisticated ligands to stabilize these non-precious metal catalysts highlight the challenges faced in this field. Finding alternative strategies that minimize both costs and resource consumption while maximizing effectiveness is vital for the future of photocatalysis.
A breakthrough in this realm has emerged from a research team led by Professor Christoph Kerzig at Johannes Gutenberg University Mainz (JGU). Their innovative approach involves the straightforward assembly of high-performance dyad photocatalysts using two commercially available salts. By exploiting electrostatic interactions between the photoactive units, the team conceived a method of forming stable ion pairs that enhance synergistic interactions. This novel technique is akin to the interaction between sodium and chloride ions in table salt, presenting a remarkably simplistic yet effective pathway to create efficient photocatalysts.
Matthias Schmitz, a key author of the study, emphasizes the practicality of this methodology. “Rather than complicating the process, we leverage established photocatalysts and add cost-effective additives that substantially boost performance,” he explains. Such an uncomplicated approach opens the doors to optimizing existing metal-based photocatalysts, potentially leading to a significant reduction in the quantity of catalysts needed for effective reactions.
The research team employed advanced spectroscopic techniques and large-scale laser devices to meticulously dissect and optimize the photocatalytic processes. They focused on the pivotal steps of light absorption by metal complexes and the subsequent activation of targeted molecules. Initial trials involving their novel class of Coulombic dyads have shown promising results, particularly in reactions forming new carbon bonds and light-induced oxygenation of wood-based substrates. Impressively, these new dyads outperformed traditional catalysts, many of which are more expensive.
This advancement highlights the capability to harness and convert sunlight and LED-generated light into valuable chemical products more effectively than ever before.
The implications of the findings are far-reaching. The realization that the solvent used can significantly impact the designed dyads suggests a customizable approach to photocatalysis. By strategically combining various photoactive ions within a solvent-based framework, researchers can optimize the efficiency of light-driven chemical reactions. This toolbox approach could result in a versatile array of photocatalysts tailored for specific industrial applications.
The researchers have high hopes that this work will pave the way for industrial photoreactions, facilitating the scale-up of production processes reliant on these innovative photocatalysts. As research progresses, the potential for cost-effective, efficient photocatalysis shifts from theoretical to practical, positioning this technology as a cornerstone for sustainable chemical processes in the future.
The development of Coulombic dyads signifies a significant step forward in photocatalysis, providing a compelling argument for harnessing simpler methodologies to revolutionize this field. Future research will undoubtedly continue to expand upon these discoveries, fostering advancements that could transform the photocatalytic landscape and beyond.

Leave a Reply