In the quest for sustainable and efficient chemical processes, researchers are turning their attention to dinitrogen (N2), a molecule that constitutes approximately 78% of the Earth’s atmosphere. Despite its prevalence, harnessing dinitrogen for chemical synthesis has proven to be a significant challenge due to the exceptionally strong triple bond that holds the two nitrogen atoms together. This bond poses a barrier to its direct use in chemical reactions, necessitating innovative approaches to unlock its potential in the synthesis of valuable industrial compounds such as polymers and pharmaceuticals.
Challenges in Traditional Approaches
The traditional method of employing dinitrogen in the production of alkyl amines typically involves the Haber-Bosch process, which first converts dinitrogen into ammonia. This ammonia then undergoes further reprocessing to synthesize alkyl amines. The process demands high energy inputs and additional steps, often including the activation of alkenes, which further complicates the reaction pathway. The reliance on such labor-intensive methodologies not only hinders efficiency but also raises concerns regarding the overall environmental sustainability of chemical manufacturing.
Takanori Shima, a prominent researcher at the RIKEN Center for Sustainable Resource Science, highlights a critical flaw in existing methods: “Synthesizing these nitrogen and carbon sources consumes a lot of energy.” The need for alternative strategies is pressing, as the drive for greener and more efficient industrial practices becomes increasingly vital.
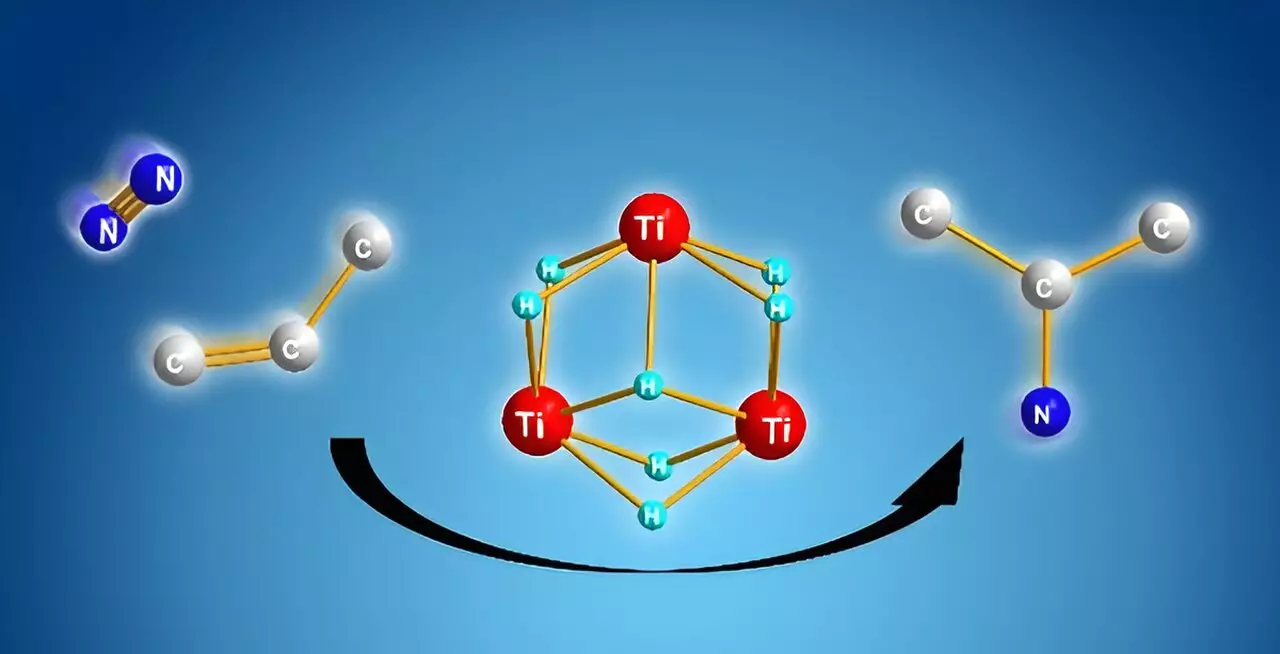
Shima and his research team have sought to develop a more streamlined method that bypasses the inefficiencies associated with traditional practices. Their breakthrough comes in the form of titanium polyhydrides—complex formations consisting of titanium atoms interconnected by hydrogen atoms. This innovative approach allows the research team to utilize the highly reactive nature of titanium polyhydrides to facilitate chemical transformations that were previously deemed difficult or impossible.
The discovery that titanium polyhydrides can react vigorously with stable small molecules such as dinitrogen marks a significant step forward. Shima explains that their investigations demonstrated how titanium polyhydride units are capable of cooperating to convert dinitrogen and alkenes directly into alkyl amines, circumventing the need for ammonia and the laborious conditioning of alkenes.
The mechanics behind this transformation are equally intriguing. Upon reacting alkenes with titanium polyhydride, the alkenes become activated. Remarkably, following this initial reaction, numerous titanium-hydride units remain unreacted and available for further interaction. When the researchers introduce dinitrogen into the mix, free titanium-hydride components work in synergy to cleave the nitrogen-nitrogen bond, subsequently linking the activated components to form a stable nitrogen-carbon bond.
Computational studies further elucidate this process, revealing that the reaction’s energetics favor the formation of nitrogen-carbon bonds over alternative pathways involving nitrogen-hydrogen or carbon-hydrogen bonds. This insight not only demonstrates the efficiency of the titanium polyhydride framework but also opens up avenues for optimizing the synthesis of desired compounds under mild conditions.
Shima and his team are now focused on harnessing this reaction as a catalytic process, potentially revolutionizing how we approach the synthesis of nitrogen-containing compounds from abundant atmospheric resources. The implications of this research are profound, paving the way for processes that could substantially lower energy consumption and reduce the environmental footprint of chemical manufacturing.
As industries worldwide grapple with the need for sustainable practices, the findings from RIKEN’s research could well represent a turning point. By innovatively leveraging the capabilities of readily available dinitrogen and titanium polyhydrides, we may be on the cusp of a new era in chemical synthesis—one that aligns with the principles of sustainability while meeting the demands of modern industry. The potential is vast, and as we look to the future, the focus will be on transitioning these laboratory insights into practical applications that can benefit both industry and the environment.

Leave a Reply