Click chemistry has revolutionized the landscape of molecular synthesis, offering pathways for creating a wide array of compounds with remarkable efficiency. Characterized by its high selectivity and rapid reaction time, click chemistry has found applications in diverse fields, from pharmaceuticals to materials science. With the continuous push towards sustainable practices, researchers have sought to refine existing methods and pioneer novel approaches that align with environmental goals.
Recent advancements in the synthesis of sulfonyl fluorides have emerged as a significant milestone within click chemistry. Historically, the synthesis of these desirable compounds involved the use of hazardous substances such as sulfur dioxide difluoride (SO2F2) or potassium hydrogen fluoride (KHF2), both known for their toxicity and handling difficulties. This posed challenges not only for chemists but also raised environmental concerns regarding the by-products generated during the reactions.
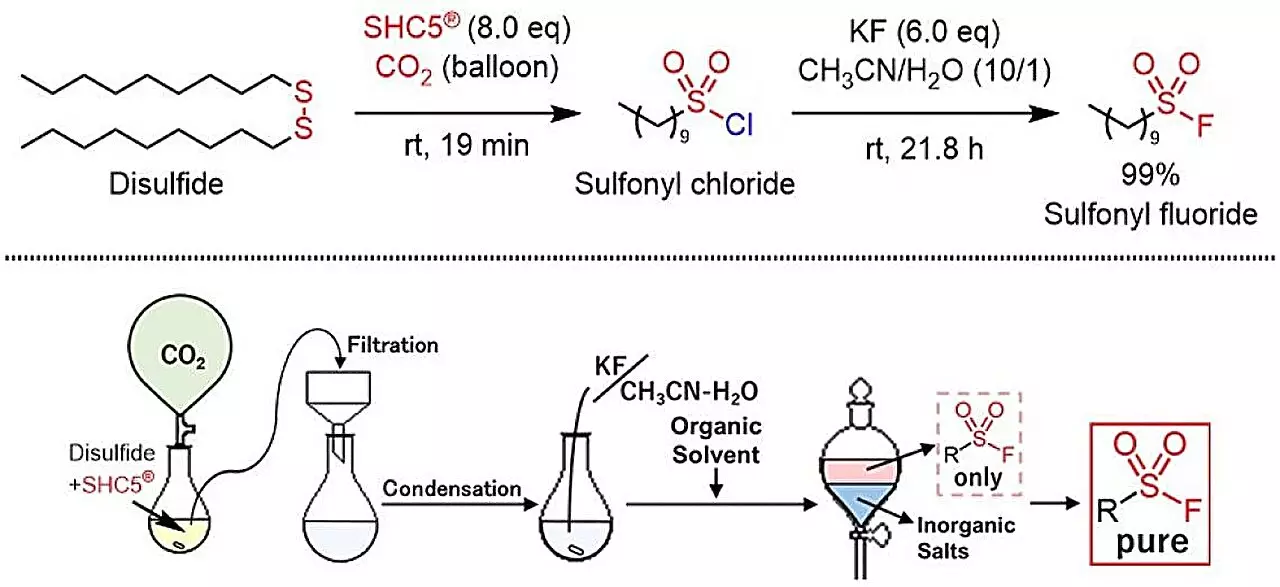
In a groundbreaking study published in ACS Sustainable Chemistry & Engineering, researchers have introduced a novel method using SHC5 and potassium fluoride (KF) to convert thiols and disulfides directly into sulfonyl fluorides. This approach marks a critical transition towards greener chemistry practices, yielding uncomplicated by-products: sodium chloride (NaCl) and potassium chloride (KCl). Such a transition toward environmentally benign synthesis emphasizes the importance of reducing toxicological risks associated with chemical processes.
The newly developed synthetic protocol is praised for its simplicity and scalability, enabling the creation of sulfonyl fluorides that contain various groups—be it aromatic, aliphatic, or heterocyclic. This method not only ensures safety and cost-effectiveness but also aligns seamlessly with today’s emphasis on sustainable development goals (SDGs). By minimizing hazardous waste and utilizing accessible reagents, this chemical process showcases how innovation can lead to practical applications that benefit both industry and the environment.
The versatility inherent in this new approach allows for a tailor-made synthesis of a broad range of sulfonyl fluorides, promising enhanced utility across multiple fields. As researchers continue to explore the implications of this methodology, its potential impacts on pharmaceutical development and material sciences become increasingly evident—a demonstration of chemistry’s capacity for innovation driven by ecological consciousness.
The successful integration of green chemistry principles into the synthesis of sulfonyl fluorides represents a pivotal development in the field of synthetic chemistry. Researchers Masayuki Kirihara, Shinobu Takizawa, and Mohamed S. H. Salem highlight not only the significance of creating functional compounds for practical applications but also the urgent need for environmentally considerate reaction processes. This advancement emphasizes that the future of chemistry lies not only in efficiency and yield but also in harmony with nature, embodying a critical step forward in the continuing endeavor to balance scientific progress with ecological responsibility. The path forward in chemical synthesis is clear: prioritize safety, sustainability, and innovation.

Leave a Reply