Ammonia (NH3) is not merely a compound; it serves as a backbone for various industries, notably in agriculture for fertilizer production and in chemical processes. The global ammonia market is a colossal entity, encompassing around 175 million metric tons with an estimated valuation of $67 billion. This underscores ammonia’s indispensable role in sustaining food production and facilitating industrial innovation. Furthermore, as the world increasingly pivots towards sustainable energy solutions, ammonia is emerging as a crucial high-energy-density carrier for hydrogen—an element pivotal for the future of energy.
However, the traditional methods of ammonia synthesis are marred by energy inefficiency and significant carbon dioxide (CO2) emissions. Predominantly, the Haber-Bosch process employs fossil fuel-derived hydrogen, thus contributing to greenhouse gas emissions. This raises the question: can we develop methodologies that minimize environmental impacts while still meeting global demand?
A promising pathway has emerged from ongoing research at Tohoku University’s Advanced Institute for Materials Research (WPI-AIMR). The initiatives spearheaded by Hao Li and his research team focus on the innovative electrochemical conversion of nitrates (NO3-) into ammonia. This novel approach proposes a shift from traditional nitrogen sources and leverages the superior properties of nitrates, providing an expedient method for ammonia synthesis.
One of the fundamental advantages of nitrate reduction over traditional nitrogen reduction is the lower dissociation energy required for its resolution. Additionally, nitrates possess inherently higher water solubility, making them much more manageable as starting materials. Not only does this method promise to streamline production efficiency, but it also addresses the prevalent environmental issue of nitrate accumulation in water bodies—a double-edged benefit that could rejuvenate ecosystems.
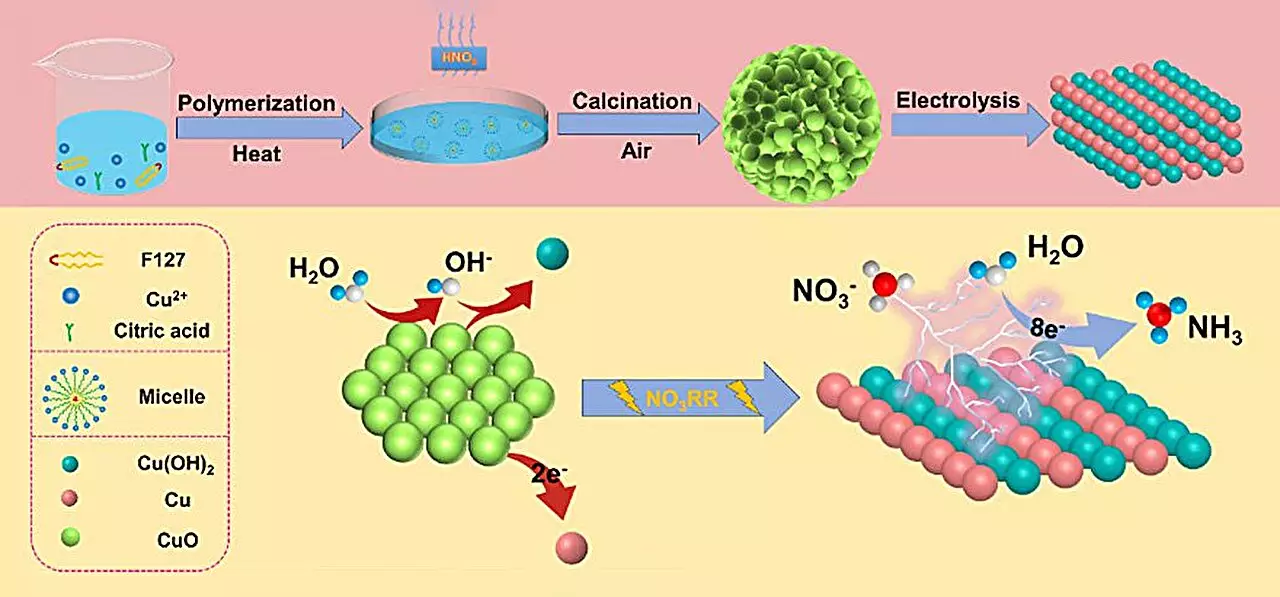
Central to this research is the synthesis of a specialized catalyst composed of copper (II) oxide (CuO). The design intricacies involve accumulating tiny particles with oxygen-rich vacancies, promoting a remarkable enhancement in ammonia yield. During experiments, this catalyst exhibited an impressive production rate of 15.53 mg h-1 mgcat-1, coupled with a Faraday efficiency of 90.69% at a controlled voltage of -0.80 V against a reversible hydrogen electrode.
The research team demonstrated that the high catalytic performance wasn’t merely coincidence—it stemmed from structural and phase changes of the catalyst during the nitrate reduction reaction (NO3RR). As elucidated by co-author Qiuling Jiang, the transformation from CuO to a hybrid Cu/Cu(OH)2 state was pivotal in unlocking this efficiency. This metamorphosis not only increases the availability of active sites for reaction but also facilitates better electron transfer processes, thereby optimizing ammonia production.
To dissect the underlying mechanisms governing this catalytic behavior, density functional theory (DFT) calculations were employed. These theoretical assessments illuminated how the formation of Cu(OH)2 directly lowers the energy requirements for nitrate adsorption, creating a more favorable reaction environment. Importantly, the Cu(OH)2 phase also acts to suppress competing hydrogen evolution reactions, which often dilute the efficiency of ammonia production.
This aspect of the research signifies a notable contribution to the fields of catalysis and electrochemistry: it presents a new framework for the development and optimization of copper-based catalysts. By utilizing insights garnered from the experimental and theoretical analyses, researchers are poised to streamline future ammonia synthesis methods.
The trajectory of this research is aligned with broader sustainability goals. While the breakthroughs achieved by Li and his team provide a foundation, the endeavor does not conclude here. The next steps involve exploring the variables that impact phase transitions of the catalyst during nitrate reduction. Such investigations aim to amplify the catalyst’s stability, activity, and selectivity, steering us towards a future where sustainable ammonia production is not just a distant possibility, but a present-day reality.
The advancement of electrochemical conversion techniques heralds a transformative era in ammonia synthesis. As the research community rallies to enhance these catalytic systems, the vision of reducing carbon emissions while fulfilling industrial ammonia demands is becoming increasingly attainable. This pioneering work stands as a testament to the potential of innovative approaches in reshaping the global landscape of food production and energy utilization.

Leave a Reply