The immunoproteasome plays a pivotal role in the immune response, functioning as a molecular guardian that ensures the body can efficiently identify and respond to pathogens, including bacteria and viruses. By breaking down foreign proteins, the immunoproteasome facilitates the presentation of these molecular signatures to immune cells, enabling them to mount a targeted and informed counterattack. However, in cases of autoimmune diseases, the malfunctioning of this system leads to an overactive immunoproteasome that mistakenly attacks the body’s own tissues, triggering painful and debilitating immune disorders.
Understanding this dual role of the immunoproteasome is essential for researchers aiming to find ways to modulate its activity. The challenge lies in selectively inhibiting this complex without interfering with the normal functions of other proteasome variants, which are responsible for essential cellular processes such as protein turnover and waste management. This selectivity is crucial, as any untargeted inhibition could lead to severe side effects and broader systemic issues.
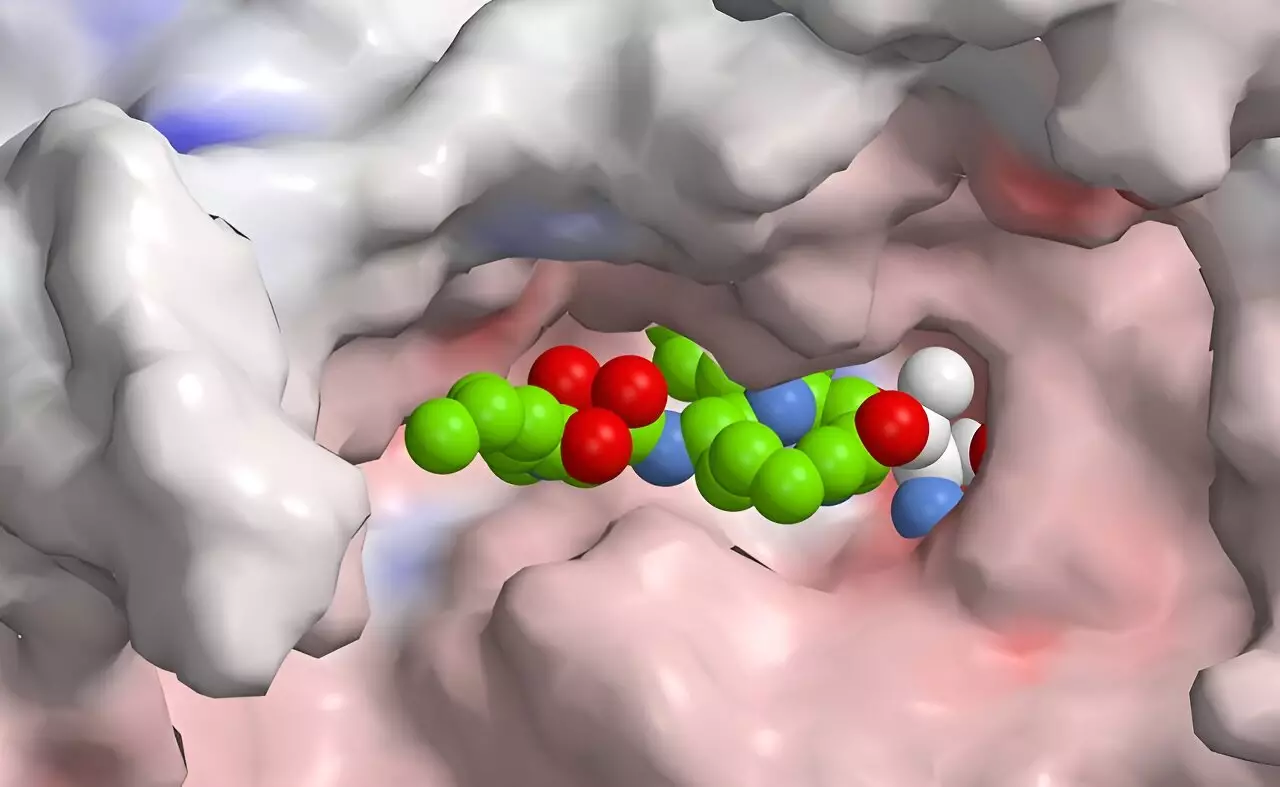
Researchers led by Helge Bode at the Max Planck Institute for Terrestrial Microbiology have recently achieved a significant milestone in this field. They have developed a novel approach to manipulate the production of natural bacterial compounds, leading to the creation of a selective inhibitor for the immunoproteasome. Their work hinges on advanced techniques in synthetic biology that allow for the construction of enzyme complexes capable of producing hybrid drugs that target specific pathways.
The key innovation in this research is the synthesis of a peptide-polyketide hybrid, a compound formed by fusing two distinct types of naturally occurring compounds. This approach is inspired by the natural occurrence of such hybrids in certain bacteria, notably those that target pests or pathogens. The presence of docking sites in thiolation domains of both non-ribosomal peptide synthetases and polyketide synthases enables the creation of such hybrids, expanding the repertoire of potential therapeutic agents.
This development has profound implications for therapeutic strategies against immune-related disorders. By combining components from peptides and polyketides, the researchers are tapping into a previously underutilized area of drug design that could yield compounds with heightened specificity for the immunoproteasome. One class of substances, known as syrbactins, provides a compelling foundation for this work. These compounds inhibit proteasomal functions in plant and insect species effectively, leading to cell death through the clogging of waste disposal pathways. Their mechanistic action is being reworked to target similar pathways in human immunoproteasome functioning, thereby holding promise as potential cancer therapies.
Despite these advances, Bode acknowledges that the newly engineered compound is not yet perfectly selective. The goal is to build on this foundation, employing computational methods and high-throughput screening to refine these inhibitors further. By leveraging technology to simulate potential interactions and outcomes, researchers can optimize compounds in silico before progressing to experimental validation.
While the new strategies developed by Bode’s team are laying the groundwork for more effective drugs, the journey is fraught with challenges. The fine balance of efficacy versus side effects is crucial, as greater potency often comes with an increased risk of unwanted reactions. Therefore, ongoing research will need to focus not only on improving selectivity but also on understanding the broader biological implications of inhibiting the immunoproteasome.
As ongoing studies shed light on the nuanced operation of the immunoproteasome and its pathways, the next generation of drugs could revolutionize the treatment of autoimmune diseases and cancers. The prospects are bright for more targeted therapies that minimize collateral damage to healthy cellular functions while effectively regulating immune responses. The work of Bode and his colleagues represents a significant step forward in harnessing the potential of natural substances for precision medicine, setting the stage for a new era in therapeutic innovation.

Leave a Reply