In recent years, the global requirement for sustainable agricultural practices and cleaner energy sources has intensified the search for innovative methods of ammonia production. Researchers have recently heralded a major advancement in catalysts for the electrochemical nitrate reduction reaction (eNO3RR), an essential process that can convert nitrate into ammonia. As outlined in a study published in ACS Nano, this breakthrough has profound implications for sustainable energy production, agricultural efficiency, and industrial applications.
Ammonia plays a vital role in food production as a primary ingredient in fertilizers, while it also emerges as an attractive candidate for zero-carbon fuel due to its high energy density and clean combustion characteristics. Moreover, ammonia benefits from existing storage and transportation infrastructures, indicating its practicality in addressing global energy demands. Traditional ammonia production, based on the Haber-Bosch process, however, presents challenges, chiefly its substantial energy consumption and its contribution to approximately 1.8% of global CO2 emissions. Thus, the need for alternative methods that are both energy-efficient and environmentally friendly has never been more pressing.
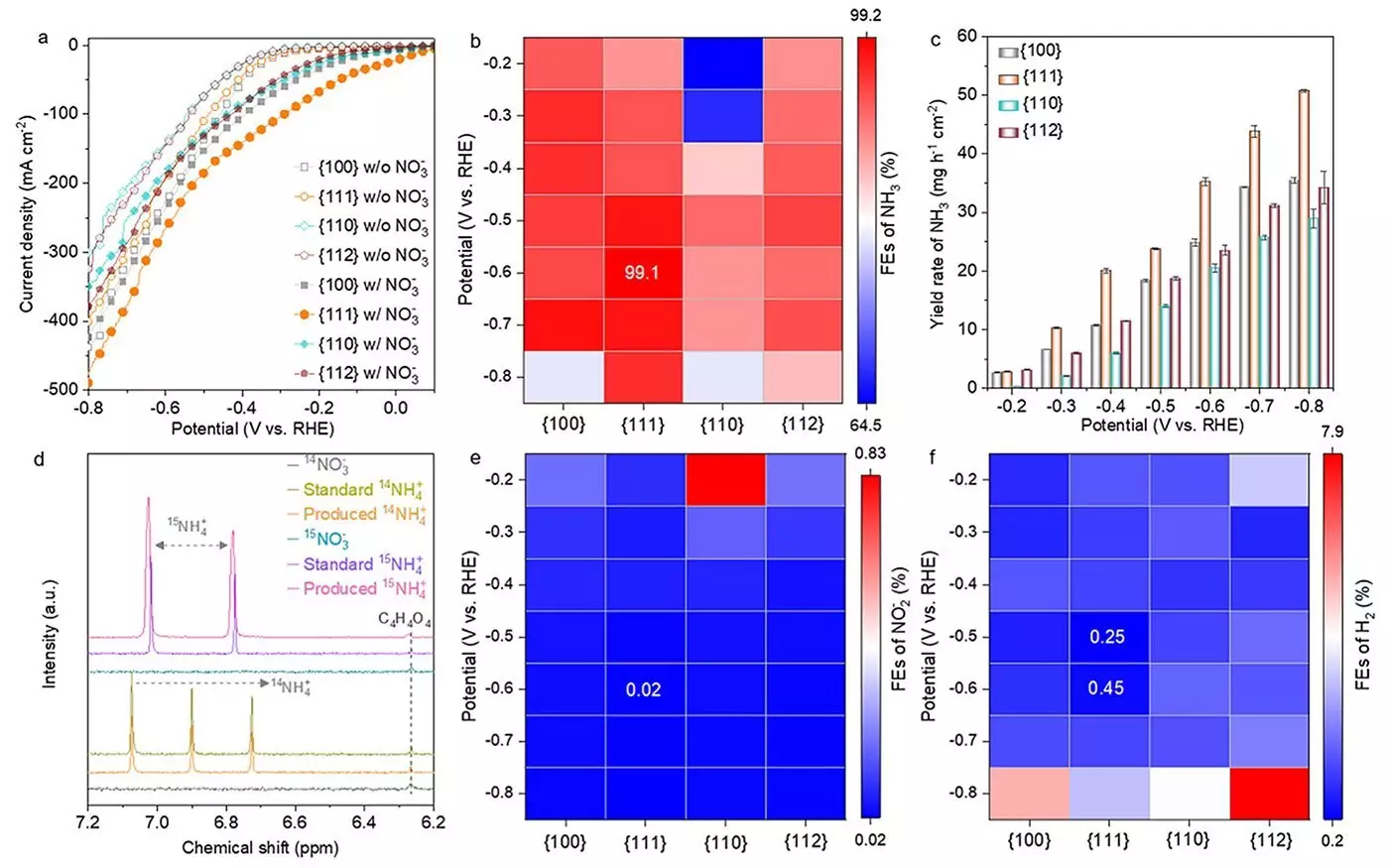
The recent study zeroes in on spinel cobalt oxides (Co3O4) as significant catalysts for the eNO3RR process due to their affordability and high efficiency. The research team synthesized a range of Co3O4 nanostructures with varying crystallographic facets, specifically {100}, {111}, {110}, and {112}. This innovative approach aimed to elucidate how the distinct crystallographic properties impact the catalyst’s efficacy in ammonia generation.
The findings revealed that the {111} facet of Co3O4 significantly outperformed other facets, achieving an extraordinary ammonia Faradaic efficiency of 99.1% and a yield rate of 35.2 mg h-1 cm-2. As Dr. Heng Liu, co-first author of the study and a Specially Appointed Assistant Professor at the Advanced Institute for Materials Research (WPI-AIMR) at Tohoku University, notes, this remarkable efficiency can be attributed to the rapid formation of oxygen vacancies and Co(OH)2 on the {111} facet. These structural characteristics drastically enhance the catalyst’s overall performance during the reaction.
An equally fascinating aspect of the research is the transformation the catalyst undergoes throughout the reaction. The Co3O4 structure evolves from an original phase to one rich in oxygen vacancies, transitioning into a hybrid CO3O4–x–Ov/Co(OH)2 configuration before stabilizing into Co(OH)2. This transformation, particularly pronounced at the {111} facet, is imperative to the catalysis process and its efficacy.
Professor Hao Li, the study’s corresponding author and associate professor at WPI-AIMR, emphasizes the critical nature of these structural changes. By unlocking the mechanisms behind the catalyst’s activation and how it transitions through various phases, researchers can derive insights that aid in the design of more effective catalysts tailored for optimized performance. Such advancements may lead to catalysts with enhanced stability, selectivity, and activity, thereby mirroring a vital step towards achieving sustainable production processes.
The implications of this breakthrough extend beyond agriculture into the broader energy sector. Ammonia is increasingly recognized as a zero-carbon fuel source that holds significant promise for energy conversion and storage technologies. This capability transforms the eNO3RR process into a valuable alternative to the conventional Haber-Bosch method, allowing for the dual benefit of waste reduction and ammonia production.
As outlined by Professor Li, this research lays the groundwork for the development of advanced sustainable catalysts that can revolutionize ammonia production. Looking forward, the focus will remain on fine-tuning the final stages of the catalyst’s transformation, thereby maximizing its efficiency and effectiveness amid environmental challenges.
The study on Co3O4 catalysts marks a pivotal moment in the ongoing effort to create sustainable industrial processes. By addressing the dual objectives of ammonia generation and environmental remediation, this research contributes to global initiatives aimed at achieving carbon neutrality by 2050. As the pressure mounts for innovative, eco-friendly solutions, ongoing research and developments such as these will be crucial in heralding a new era of energy and agricultural sustainability.

Leave a Reply