In a groundbreaking discovery, a research team led by Professor Ingo Krossing at the University of Freiburg has reshaped the landscape of molecular and coordination chemistry. Through innovative experimentation, they have extended the oxidation potential of positive ions significantly, marking a pivotal advancement that could influence diverse fields, from electrocatalysis to materials science. Their published findings in *Nature Communications* unveil a comprehensive methodology that enhances the oxidation capabilities of conventional ions like Ag+ and NO+, amplifying their practical applications in chemical research.
The Importance of Oxidation Potentials
Oxidation reactions are at the heart of many chemical processes, making the ability to manipulate and enhance oxidation potentials crucial for researchers. Traditionally, the usable potential for positive ions in standard solvents is capped at +0.65 to +1.0 V vs. Fc+/0. However, Krossing’s team reported astonishing advancements, achieving potentials soaring up to +1.50 and +1.52 V. This increase opens the door for exploring previously unattainable redox reactions—particularly valuable for hard-to-oxidize substrates that have stymied advancement in synthetic capabilities.
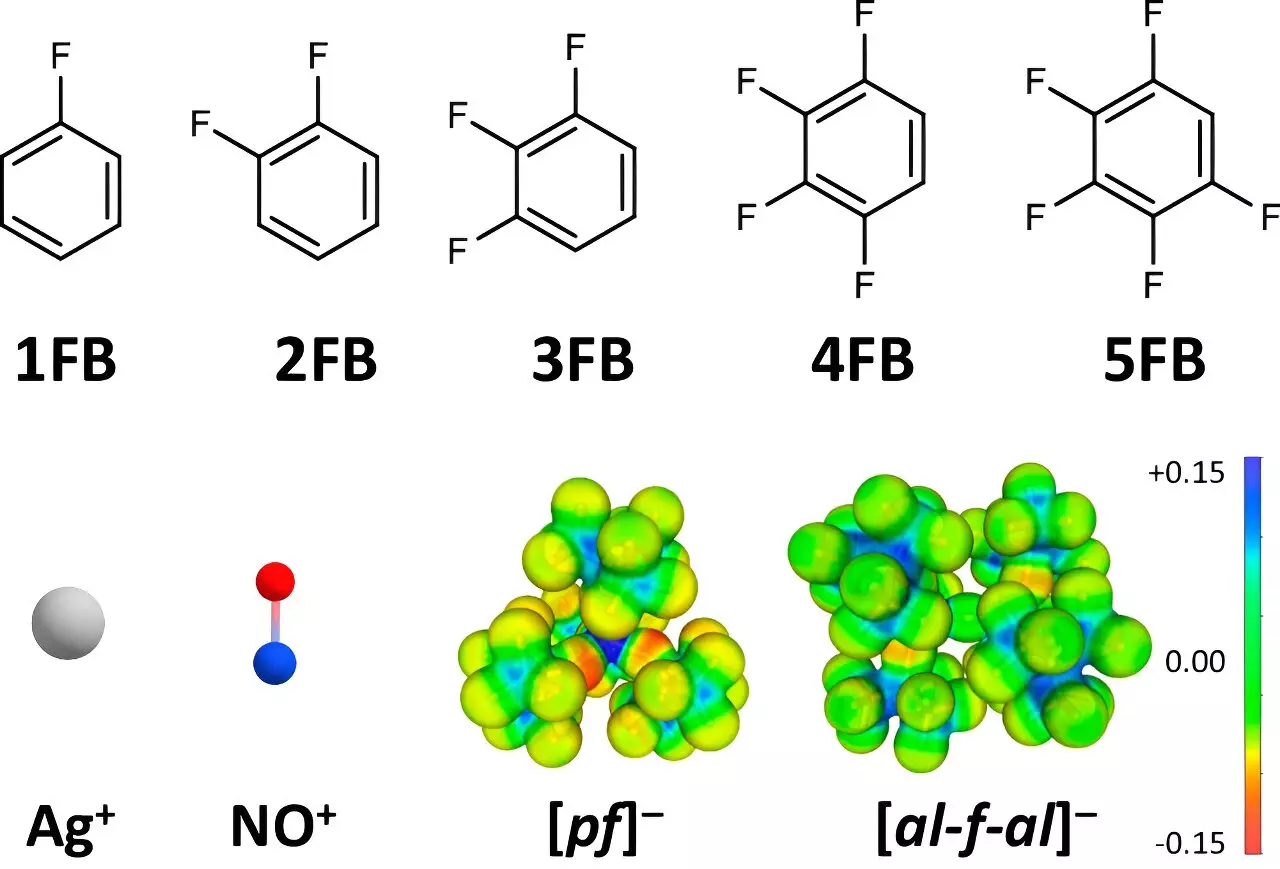
The breakthrough leverages specifically weakly interacting solvents and anions, with the research team honing in on strategically fluorinated benzene derivatives. These solvents have unique properties that render them more effective in stabilizing positive ions, thus facilitating heightened oxidation capabilities. By carefully selecting solvents with diminishing interactions at higher fluorination levels, the team struck a balance where the ionic interactions are reduced while retaining adequate solubility, maintaining higher oxidation potentials.
Weakly coordinating anions (WCAs) play a pivotal role in optimizing the oxidation of dissolved positive ions. By minimizing the interference between the ions and their surrounding molecular environment, these WCAs mitigate the reduction of oxidation potential typically observed. This carefully crafted environment allows Ag+ and NO+ ions to operate more effectively, enhancing their role as oxidizing agents that can precisely remove electrons from substrates in a controlled manner.
A key element in this research was a comprehensive analysis of the dielectric properties of fluorinated aromatic compounds. Collaborating with Dr. Johannes Hunger from the Max Planck Institute for Polymer Research, the team investigated dielectric constants—a critical factor influencing solvent behavior in chemical reactions. Their findings revealed that compounds with two to four fluorine atoms exhibited higher dielectric constants than traditional solvents like dichloromethane or acetone, underscoring the superiority of these novel solvents in achieving enhanced electrochemical reactions.
In addition to electrochemical evaluations, the research team employed single-crystal X-ray diffraction techniques to elucidate the solid-state structures of the positive ions in conjunction with their solvents. The results indicated that interactions diminish with increased fluorination, leading to nearly unperturbed ionic species that pave the way for these elevated oxidation potentials. Co-author Dr. Malte Sellin emphasized that this interaction dynamic is crucial for facilitating previously impossible reactions, adding that the ramifications of these findings extend into uncharted territories for research and practical applications.
The recent advancements spearheaded by Professor Krossing and his team establish a new frontier in the study of oxidation potentials. By harnessing the unique properties of weakly coordinating anions and fluorinated solvents, the scientists have not only augmented the oxidation efficacy of well-studied ions but have also set the stage for innovative applications in electrocatalysis and new material formation. This research promises to invigorate the fields of inorganic and synthetic chemistry, opening many avenues for future exploration, leading to breakthroughs that can shape the future of chemical reactions and engineering.

Leave a Reply