Advancements in energy storage technology are critical as the world increasingly shifts towards renewable energy sources. Among these innovations, solid-state batteries have attracted considerable attention due to their potential advantages over traditional liquid electrolyte batteries. While the outlook for solid-state electrolytes has been promising for decades, recent research from the University of Illinois Urbana-Champaign posits that the key to optimal performance may lie in the molecular structure of the materials used.
Current liquid electrolytes have inherent challenges, including safety risks associated with leaks and flammability. These drawbacks have spurred scientists to explore solid-state alternatives, which promise enhanced safety and stability. However, the quest to develop solid polymer electrolytes that can rival the performance of liquid counterparts has necessitated groundbreaking approaches. Researchers are now realizing that the arrangement of molecules within the polymer matrix is crucial for achieving significant improvements in ionic conductivity.
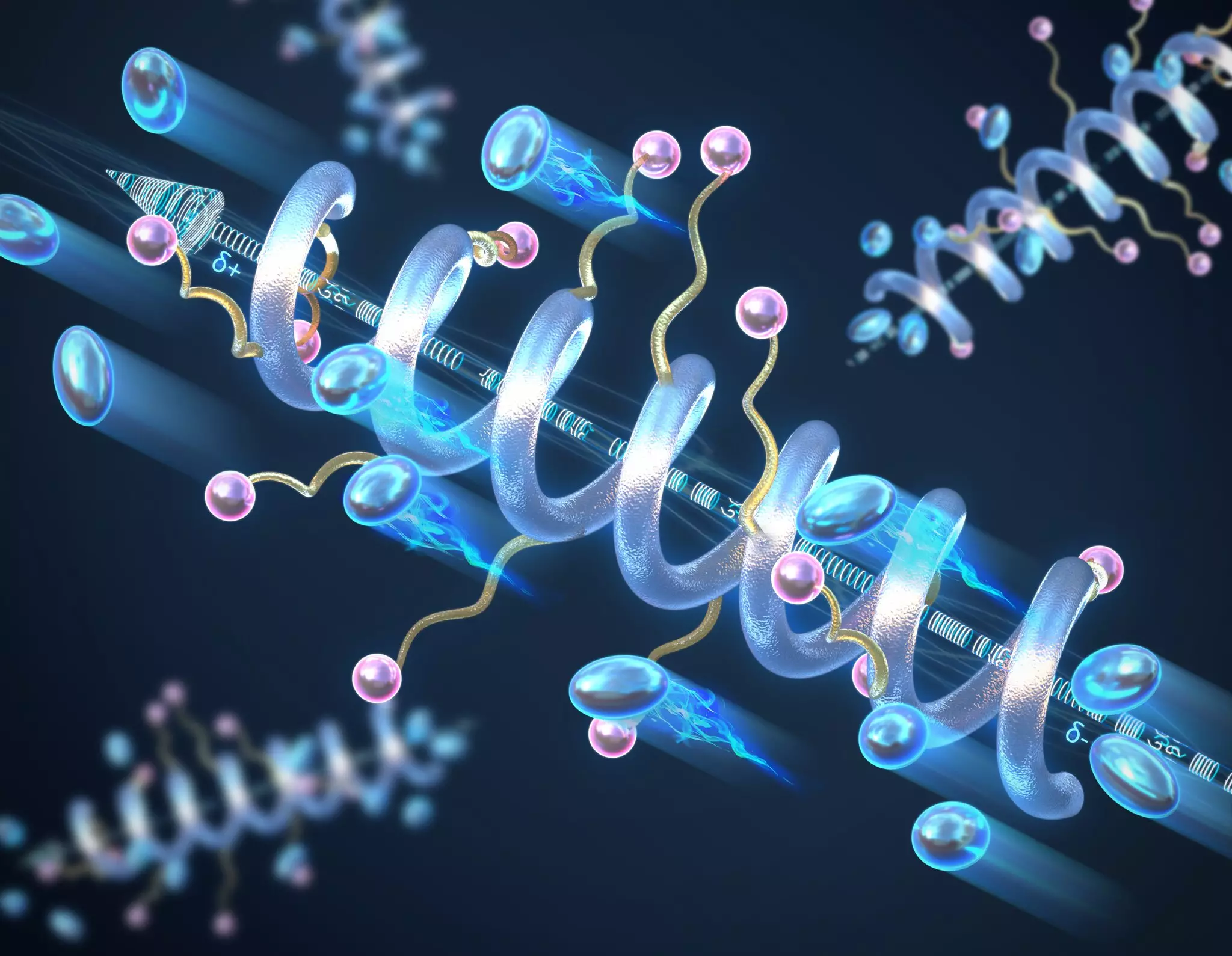
In the recent study published in *Nature Materials*, a team led by Professor Chris Evans has unveiled the transformative potential of helical secondary structures in peptide-based polymers. The findings indicate that arranging these polymers into a helical formation markedly boosts ionic conductivity compared to traditional random coil configurations. Pauling’s classic contributions to molecular biology find resonance in this study, where helices inspire innovations in battery technology.
Helical formations not only enhance conductivity but also provide exceptional thermal and voltage stability, positioning these materials as viable candidates for next-generation batteries. The research highlights a fascinating interplay between polymer architecture and its electrical properties, suggesting that utilizing biological principles can drive advancements in non-biological applications.
The significance of the helix lies in its ability to create a macrodipole moment throughout the polymer chain. In simpler terms, as helices form, small dipole moments from each individual peptide accumulate to produce a larger, coherent dipole. This structural arrangement contributes to an impressive dielectric constant—the material’s ability to store electrical energy—thus facilitating efficient charge transport. The longer the peptide helix, the greater the resultant conductivity, emphasizing the importance of polymer design.
Moreover, Professor Evans notes that these helical polymers demonstrate resilience in high-temperature and high-voltage scenarios. Such robustness is not typically observed in conventional polymers, where random coil structures are prone to degradation. The stability of these helices could significantly extend the lifespan of the energy storage systems based on them, addressing one of the critical issues with current battery technologies.
Another pivotal aspect of this research is the environmental impact of using peptide-based polymers. Unlike traditional battery materials that can contribute to hazardous waste, these helices can be broken down into their constituent monomer units at the end of their lifecycle through enzymatic or acidic processes. This ability to decompose and recover starting materials presents an appealing solution for addressing environmental concerns tied to battery disposal and reinforces the potential for circular economy practices in energy storage technologies.
The exploration of helical peptide polymer electrolytes marks an important milestone in the development of solid-state batteries. The findings from the University of Illinois Urbana-Champaign provide a promising glimpse into how advancements in materials science can contribute to safer, more efficient, and sustainable energy storage solutions. As we face global challenges associated with energy supply and environmental degradation, the innovative integration of biological structures into energy technologies may pave the way for the next generation of batteries that meet the needs of an increasingly tech-driven society. As researchers continue to investigate and refine these materials, a future with safer and more effective energy storage solutions is within reach.

Leave a Reply