Anorexia nervosa is a multifaceted mental health disorder that manifests through restrictive eating patterns, an intense fear of gaining weight, and distorted body image perceptions. Individuals suffering from this debilitating condition not only struggle with emotional and psychological challenges such as severe anxiety and depression but also face serious nutritional deficiencies that can jeopardize their physical health. As medical research continues to probe the underlying causes and mechanisms of anorexia nervosa, recent findings shed light on the potential role of neurotransmitter function, offering avenues for innovative treatment strategies.
While the etiology of anorexia nervosa remains not fully elucidated, a notable study has pointed to changes in neurotransmitter activity, particularly within the brain. Understanding the interplay between brain chemistry and eating disorders could facilitate better therapeutic interventions. Prior research has already established correlations between anorexia and marked alterations in brain structure, as well as dysfunction of key neurotransmitters. Notably, studies focusing on the neurotransmitter acetylcholine have revealed intriguing connections in animal models, indicating a deficiency in this chemical within the striatum, a region of the brain integral to motor function and reward response.
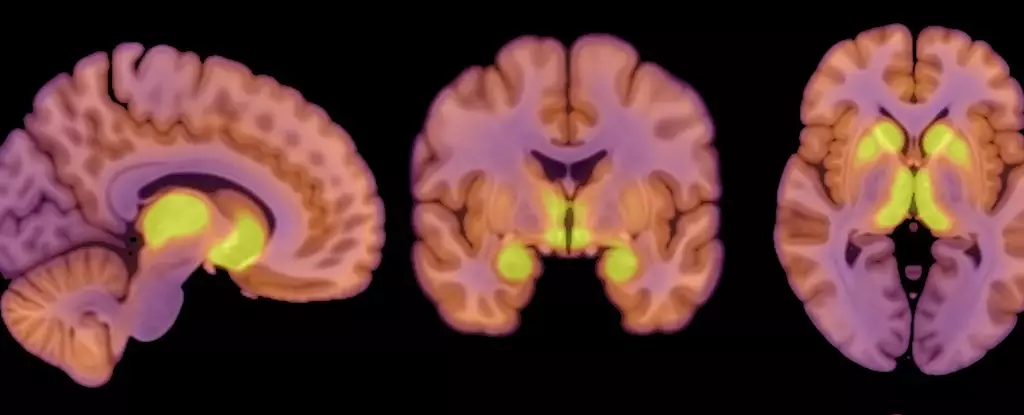
A critical advance highlighted in recent research is the identification of mu-opioid receptors (MORs), proteins integral to the brain’s opioid system, which regulates both feeding behaviors and the experience of pleasure derived from food consumption. This study, led by researchers at the University of Turku, demonstrated a significant association between higher levels of MOR availability in brain regions associated with reward processing and individuals diagnosed with anorexia nervosa.
Co-author Pirjo Nuutila, a physiologist involved in the research, articulates the importance of this finding, noting that the opioid neurotransmitter system plays a dual role in appetite regulation, both enhancing and suppressing the drive to eat. The dual nature of MORs suggests a complex balance—wherein they can regulate cravings for both food and pleasure. It raises the question of how individuals with anorexia may experience heightened reward responses in their brains, contributing to their restrictive eating behaviors.
The recent study encompassed a sample of 13 female patients, ranging from 18 to 32 years of age, each displaying a body mass index (BMI) of less than 17.5, indicative of severe underweight status. In contrast, the control group consisted of 13 healthy females, all within the same age bracket but possessing BMI values ranging from 20 to 25. This design facilitated a focused analysis of the neurochemical differences between these two populations.
Using positron emission tomography (PET) scans, researchers assessed both the MOR availability in the participants and their brain glucose uptake, acknowledging the human brain’s substantial energy demands—approximately 20% of total daily energy expenditure. Findings revealed that despite the caloric restrictions imposed by anorexia, the patients’ brains continued to maintain energy levels comparable to those of their healthy counterparts. This observation is particularly striking, indicating that the brain demonstrates protective mechanisms to preserve its function, even amidst significant physiological distress stemming from malnutrition.
The study’s results suggest an intriguing relationship between elevated MOR availability and stable brain glucose uptake in individuals with anorexia, positing the endogenous opioid system as a potential key player in the disorder. The findings propose that the brain’s opioidergic activity may reflect a neurochemical environment that incentivizes weight loss and disordered eating behaviors, creating a contrast to the down-regulated MOR systems observed in obesity.
However, the findings do come with caveats; the researchers acknowledge the limitations surrounding the small sample size and the exclusive focus on female participants. This gender bias raises questions about the generalizability of the findings to male populations, where anorexia, though less prevalent, still poses significant health risks. Furthermore, the absence of extensive behavioral assessments leaves a gap in linking observed neurophysiological changes to specific eating patterns and habits, highlighting the need for broader studies that can draw deeper connections.
Despite the limitations of the study, the connection drawn between neurotransmitter function, brain structures, and anorexia nervosa lays the groundwork for future research endeavors. Understanding the neurobiological underpinnings of anorexia may eventually lead to more effective treatment approaches that can directly target the root causes of this serious mental health condition.
As inquiries into the complex interplay between brain function, eating behavior, and emotional health continue, it becomes increasingly apparent that a multidisciplinary approach is essential for developing comprehensive treatment plans. Future investigations must consider diverse populations, larger sample sizes, and a broader array of measures to truly elucidate the intricate mechanisms involved in anorexia nervosa. In doing so, we can hope to forge a path toward recovery for those impacted by this challenging disorder.

Leave a Reply