In the face of growing environmental concerns and the pressing need for sustainable energy solutions, the scientific community has witnessed a surge in innovative methodologies aimed at making chemical production more eco-friendly and efficient. A pioneering electrochemical approach developed by a team from the Lawrence Livermore National Laboratory (LLNL) exemplifies this trend, introducing a transformative method for chemical synthesis that relies on thin-film nickel anodes. This breakthrough not only enhances energy efficiency but also significantly reduces the environmental footprint associated with traditional chemical manufacturing processes.
The utilization of thin films in this research plays a critical role in achieving consistent and reliable experimental results. LLNL postdoctoral researcher Aditya Prajapati highlights how thin-film anodes facilitate a clearer understanding of catalytic processes. By providing a homogeneous surface, these thin films eliminate the variability associated with porous and rough structures, thereby allowing scientists to accurately assess the catalytic properties of materials. This methodical approach to studying catalysts empowers researchers to optimize chemical reactions, directly translating into improved production processes that align with sustainability goals.
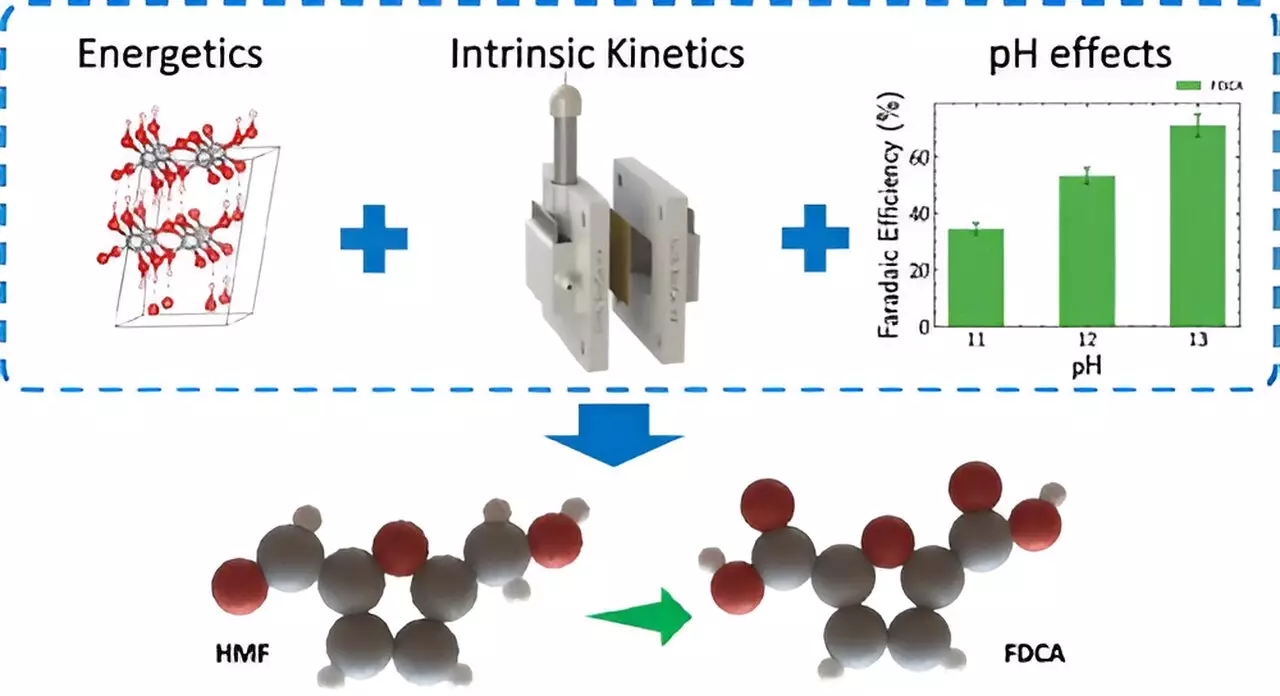
One of the significant challenges with current electrolyzer technology is the inefficiency stemming from the oxygen evolution reaction at the anode. The LLNL team tackled this issue head-on, unveiling a method that replaces this conventional process with biomass oxidation. This innovative switch could result in energy savings exceeding 50%, thereby enhancing both the efficiency of chemical production and the sustainability of biomass conversion practices. By employing 5-Hydroxymethylfurfural (HMF), a compound derived from biomass, the team’s method converts it into 2,5-Furandicarboxylic acid (FDCA), a crucial component in the manufacturing of bio-based plastics.
By integrating renewable feedstocks into the chemical production landscape, this electrochemical method advances the principles of a circular economy, promoting the reduction of dependence on fossil fuels. Nitish Govindarajan, another key contributor to this project, emphasizes the cleaner and more energy-efficient nature of this approach, contrasting it with traditional methods that typically generate toxic waste and require elevated temperatures. The potential for a zero-carbon footprint looms large, especially when this electrochemical conversion process is paired with renewable energy sources.
The research conducted by the LLNL team provides a promising glimpse into the future of chemical manufacturing, where efficiency meets sustainability. With the capacity to lower carbon emissions and petroleum dependency while harnessing renewable resources, this electrochemical method stands as a testament to the advances in green chemistry. Collaborative efforts across institutions, including contributions from Université de Montréal and the University of Bonn, further enrich this field of study. As researchers continue to refine these technologies, the opportunities for cleaner chemical production are bound to expand, paving the way for a more sustainable industrial landscape.

Leave a Reply