Bacteria have developed an impressive array of strategies to protect themselves against environmental threats, including the immune systems of their hosts. One of the most effective defenses employed by many bacterial pathogens is the formation of a capsule — a shell comprised of polysaccharides that not only shields the bacterium from physical damage but also helps it evade detection by the host’s immune responses. Understanding the mechanisms behind capsule formation is critical for developing targeted treatments that could disarm these pathogens, making them vulnerable to immune attack.
Capsular polymers, the sugar chains that form bacterial capsules, play a pivotal role in the survival of various pathogens. These structures act as protective barriers, preventing desiccation in harsh environments and facilitating bacterial persistence within host organisms. The complexity and structural diversity of these polymers are significant, varying considerably across different bacterial species. The construction of these capsules involves a series of enzymatic reactions, and disrupting the formation of these polymers could weaken the bacteria drastically, opening them up to immune system assaults.
Research has highlighted that enzymes involved in capsule biosynthesis are prime targets for therapeutic interventions. By inhibiting these enzymes, researchers could potentially develop new antibiotics or biotechnological tools for vaccine production. However, a significant knowledge gap remains regarding how these polysaccharides are anchored to the bacterial cell membrane, which is critical to both capsule formation and overall bacterial pathogenicity.
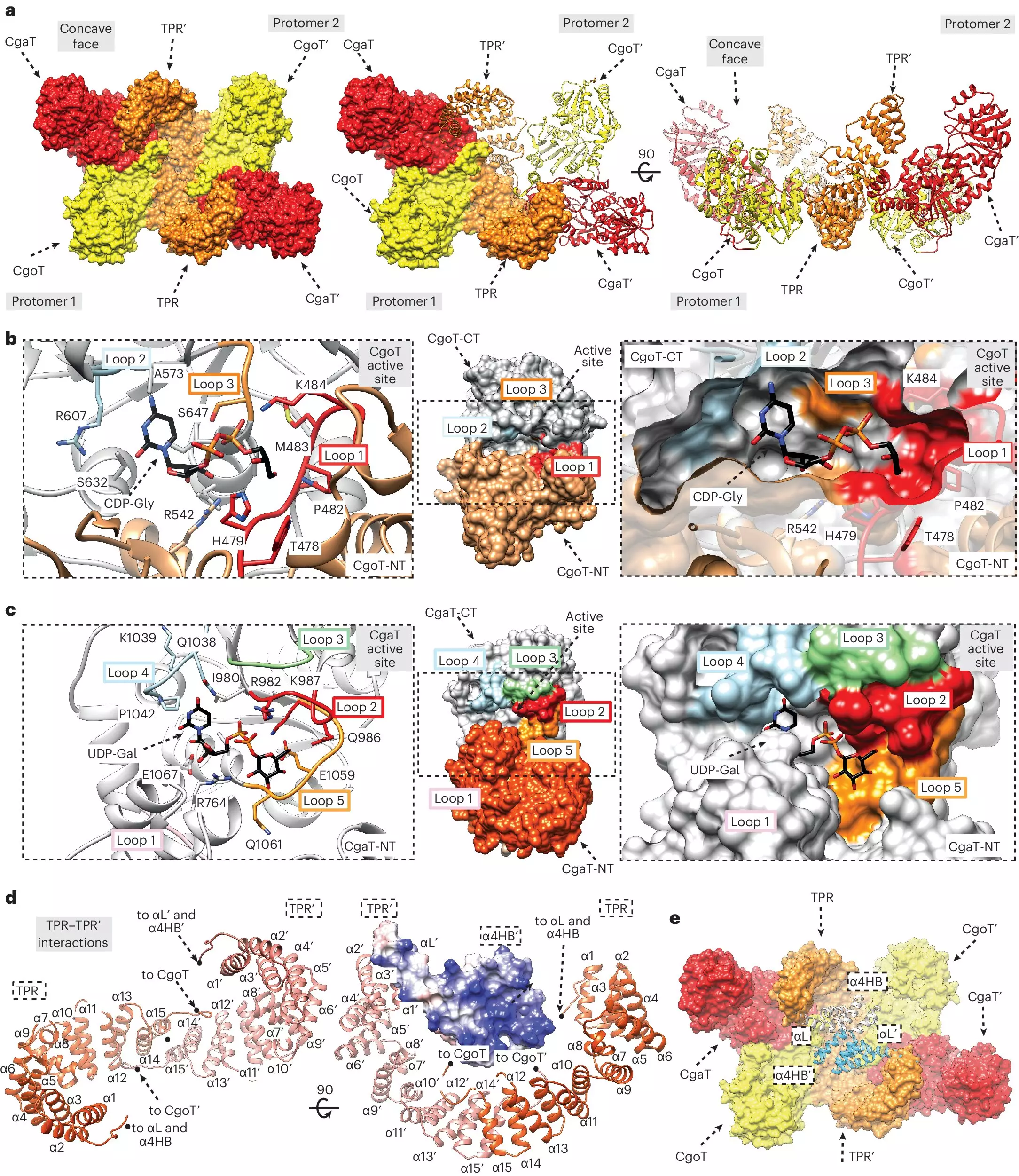
A team of international researchers, led by Dr. Timm Fiebig from the Hannover Medical School, has made important strides in this area. They have identified a previously elusive linker molecule that connects the fatty acid anchor in the bacterial membrane to the capsular polymer. This discovery represents a breakthrough in understanding the biosynthetic pathways behind bacterial capsules. Furthermore, the team characterized transition transferases—enzymes responsible for the synthesis of this linker—as potential drug targets that could facilitate the development of new antibacterial agents.
Previously, studies had documented the role of capsular polymerases in the growth of polysaccharide capsules, particularly concerning pathogens like Haemophilus influenzae type b (Hib), a bacterium recognized for causing severe respiratory tract infections and meningitis. The identification of the linker and its associated enzymes allows for a more comprehensive understanding of how capsules are constructed and highlight new avenues for therapeutic exploitation.
The research team utilized advanced chromatography techniques to purify the linker and the relevant enzymes, enabling them to elucidate their structural characteristics. The capacity of the transition transferases to elongate the linker, thereby stimulating the activity of capsular polymerases to generate longer sugar chains, was particularly illuminating. This intricate interaction is likely crucial in enhancing the protective capabilities of the bacterial capsule.
By probing these enzymatic interactions and structural distinctions, the research advances the field’s understanding of bacterial capsule biosynthesis. It challenges prior assumptions, revealing that the linker possesses a unique structure that is distinct from the polysaccharides of the capsule itself. This insight is vital for the future identification of similar enzymes across various bacterial strains, which could lead to the discovery of novel therapeutic agents with broad-spectrum efficacy.
The implications of these findings extend beyond basic research; they point towards revolutionary potential in antibiotic development. Given that different bacterial species may utilize analogous mechanisms to produce their capsular structures, targeting the transition transferases involved in linker synthesis could pave the way for drugs that act against multiple bacterial strains. The research team has observed structural similarities in the linker across several pathogens associated with serious infections, underscoring the significance of their findings.
By disabling the enzymes responsible for the linker’s formation, researchers could effectively hinder the capsule’s assembly, rendering the bacteria defenseless against the host’s immune system. This approach not only emphasizes the importance of molecular research in bacteria but also showcases a possible new frontier in the battle against infectious diseases.
Understanding the molecular underpinnings of bacterial capsule formation and its protective strategies is essential for advancing therapeutic interventions. The recent work conducted by the research team led by Dr. Fiebig marks a significant step forward in our quest to harness this knowledge for drug development. By dissecting these complex biochemical pathways, scientists are developing new strategies to combat bacterial infections and potentially save countless lives from the threats posed by antibiotic-resistant strains.

Leave a Reply