In the quest for sustainable energy solutions, the research led by Prof. Chen Changlun at the Hefei Institutes of Physical Science demonstrates that innovation is paramount in the race against climate change. Their work on cobalt-doped nickel hydroxide bipolar electrodes signals a pivotal shift in the way we approach hydrogen production through two-step water electrolysis. Traditional methods have hit roadblocks, primarily due to their inability to efficiently sync with renewable energy sources and manage the complexities of hydrogen and oxygen generation under high pressure. By addressing these limitations, the research team has set a new standard for efficiency and stability in hydrogen production.
The Limitations of Conventional Alkaline Electrolyzers
Alkaline electrolyzers, while historically significant, face considerable challenges that have stunted their wide-scale application. The mismatched dynamics with fluctuating renewable energy inputs create inefficiencies, and the mixing of hydrogen and oxygen at high pressures poses safety risks. These roadblocks necessitate the development of innovative processes that can mitigate these issues, which is where the research team’s innovative approach comes into play. By decoupling the processes of hydrogen and oxygen production through strategic electrode design, they have created a method that not only bypasses costly membrane separations but also enhances operational safety.
Innovative Materials: The Heart of the Research
The core of this groundbreaking research lies in the development of advanced materials. The adoption of a one-step electrodeposition method for crafting cobalt-doped flexible nickel hydroxide bipolar electrodes signifies a technological leap. This innovative approach not only boosts conductivity but also ensures robust electron storage capability, curbing unwanted side reactions such as parasitic oxygen production. Additionally, the exploration of non-noble metal catalysts like molybdenum-doped nickel-cobalt phosphide and plasma-induced iron composite cobalt oxide has showcased impressive durability and catalytic activity. The implications of these findings extend beyond laboratory walls, potentially revolutionizing the hydrogen production landscape.
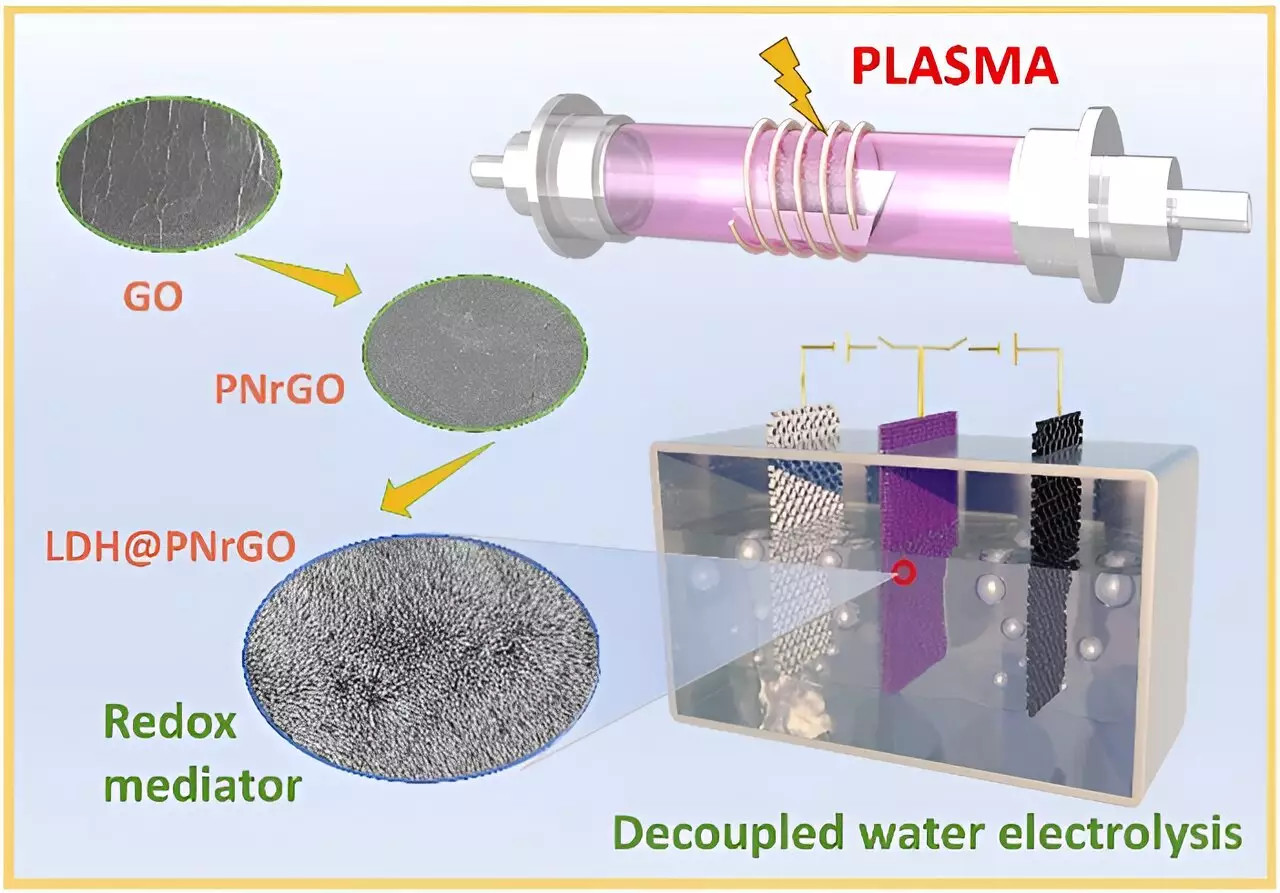
Revolutionizing Electrode Design and Functionality
Transitioning from traditional layered double hydroxide (LDH) frameworks to innovative designs using plasma technology has further enhanced performance metrics. The emergence of nitrogen-doped nickel-cobalt LDH and nitrogen-doped reduced graphene oxide/nickel-cobalt LDH has created electrodes with significantly improved electronic properties. This advancement indicates not only better energy storage capabilities but also a stable discharge performance, making the electrodes suitable for various demanding applications, from industrial setups to data centers reliant on hydrogen fuel.
A Vision for the Future
Prof. Chen Changlun’s assertion that their performance indicators for two-step water electrolysis now align with global benchmarks reflects a monumental achievement. The research team’s findings suggest that this technology could be at the forefront of large-scale hydrogen storage solutions, critical for powering future technologies such as 5G networks. As the world increasingly pivots towards renewable energy, innovations in hydrogen production like those from the Hefei Institutes of Physical Science will be crucial in establishing a sustainable energy landscape that we can rely on for tomorrow.
The commitment to transforming hydrogen production through advanced materials and innovative designs marks a powerful step forward in achieving a hydrogen-fueled future.

Leave a Reply