In the realm of chemistry, few elements have had as storied a history as gallium. Since its discovery by French chemist Paul-Émile Lecoq de Boisbaudran in 1875, this unique element has piqued the interest of scientists across various disciplines. Fast forward nearly 150 years, and researchers at the University of Auckland have made astonishing revelations about this enigmatic metal. Their work not only reshapes our understanding of gallium but challenges long-held scientific beliefs that have persisted for decades.
Gallium, with its notoriously low melting point—so low that a gallium spoon can melt in a cup of tea—has long been a staple in the realm of semiconductors. However, this recent study has delved deeper, offering significant insights into gallium’s behavior at the atomic level. This unique perspective enhances not only the scientific community’s comprehension of gallium but also its practical applications in modern technology.
The Unusual Nature of Gallium
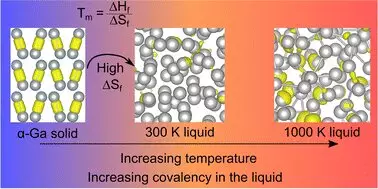
What sets gallium apart from most metals is its formation of ‘dimers’, which are pairs of atoms. In a fascinating twist, gallium is less dense in its solid state than in liquid, mirroring the behavior of ice floating on water. This is quite an anomaly within the metal category, where one usually expects solids to be denser than their liquid counterparts. Coupled with the presence of covalent bonds—where atoms share electrons—gallium’s identity diverges markedly from that of more familiar metallic elements.
Data published in the paper titled “Resolving Decades of Debate: The Surprising Role of High-Temperature Covalency in the Structure of Liquid Gallium,” challenges the accepted notions about gallium’s properties. Professor Nicola Gaston, a leading figure in this research, articulately observes that previous literature fundamentally misrepresented gallium’s behavior. This revelation compels a reassessment of the scientific community’s understanding regarding why gallium possesses such a low melting point.
Entropy and the Greater Picture
Central to this new understanding is the role of entropy, which serves as a measure of disorder. The researchers postulate that as the covalent bonds in gallium dissipate during the melting process, there is a substantial surge in entropy. This newfound freedom between the atoms enhances gallium’s mobility and contributes to its unique physical behavior. Dr. Steph Lambie, who played a vital role in this research while completing her Ph.D., painstakingly revisited decades of literature, correlating temperature data to create a cohesive narrative that informs this transformative understanding.
This meticulous approach showcases the significance of scholarly perseverance; revisiting old literature can yield groundbreaking insights that reshape entire fields. In an era where technology evolves rapidly, maintaining a clear understanding of core materials like gallium is essential for innovation.
Implications for Nanotechnology and Beyond
The implications of this research are far-reaching, particularly within the burgeoning field of nanotechnology. As scientists continue to manipulate materials at the atomic scale, a comprehensive grasp of gallium’s properties is paramount. Its utility extends from dissolving other metals to forming liquid metal catalysts capable of creating complex self-assembling structures. Previous projects have demonstrated gallium’s potential, such as crystallizing zinc into ‘snowflakes’, which illustrates how far-reaching gallium’s influence can be.
Moreover, gallium’s applications span several industries, including telecommunications, LEDs, and solar panels, making it a linchpin in contemporary technology. Surprisingly, scientists also see gallium’s relevance extending to astrobiology; its chemical properties may help unearth clues about past microbial life on Mars, showcasing the metal’s broader significance in our quest to understand life beyond Earth.
The Legacy of Gallium and the Future of Scientific Inquiry
Fundamentally, gallium embodies the spirit of scientific inquiry itself—a reminder that even well-established knowledge can be upended by new discoveries. As researchers continue to explore its properties, they unveil a myriad of possibilities and applications, prompting us to consider both the past and the future of scientific achievement. The journey of understanding gallium is a testament to the relentless pursuit of knowledge, where each discovery shines a light on the intricate dance of atoms that forge the materials we often take for granted. In this light, gallium is not just a metal; it is a symbol of exploration and innovation in the ever-evolving world of science.

Leave a Reply