Aromaticity has long been a cornerstone in the realm of chemistry, primarily associated with cyclic compounds rich in carbon. These stable structures have enchanted chemists for decades due to their unique stability and reactivity. While the concept has been predominantly carbon-centric, recent breakthroughs challenge this limitation by introducing the concept of aromaticity in entirely metallic systems. The recent study led by Prof. Dr. Lutz Greb and his team at Heidelberg University has not only isolated a bismuth metal ring but has also unveiled a promising method for stabilizing such exotic structures, marking a pivotal moment in inorganic chemistry.
The Landmark Discovery
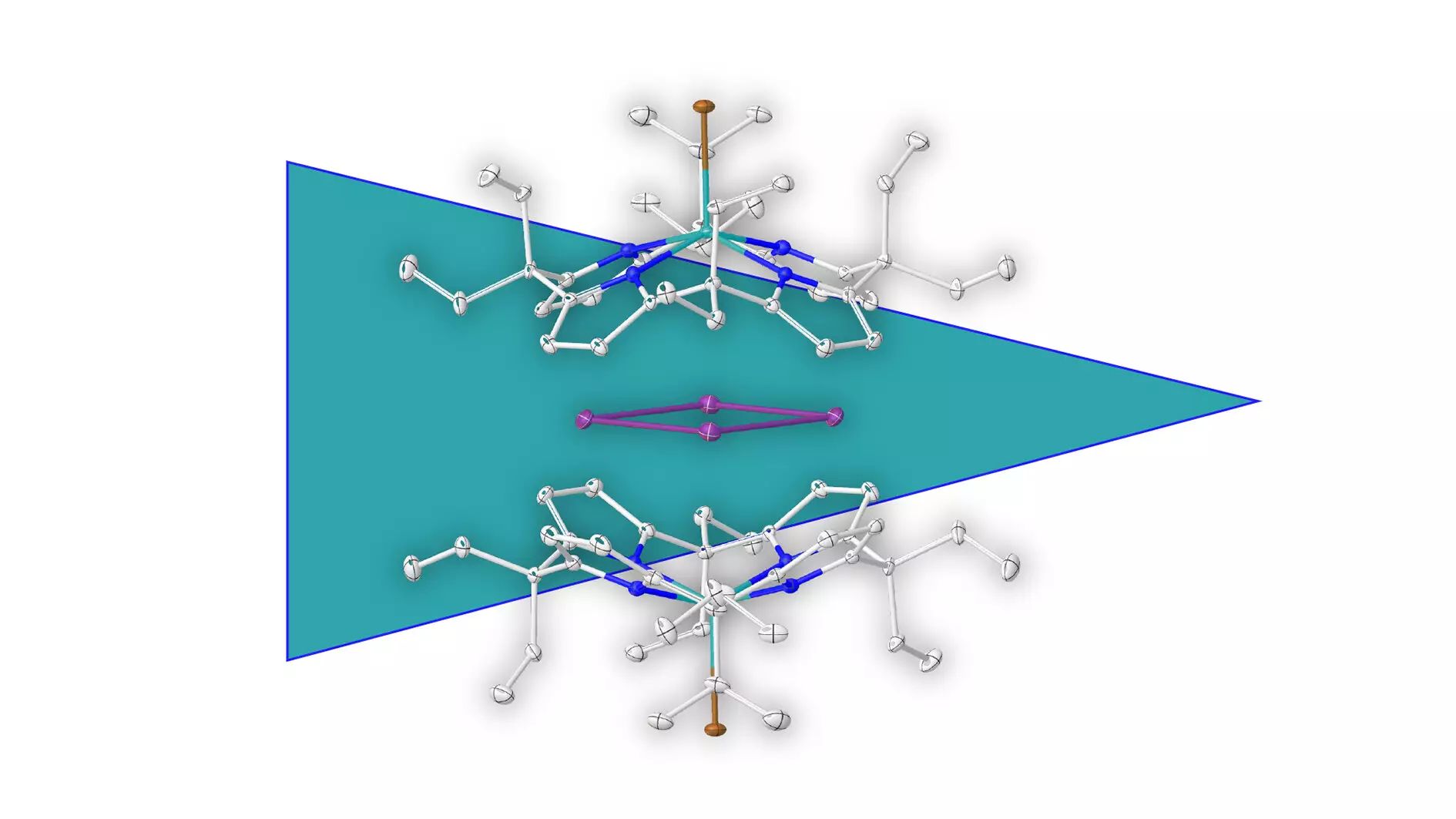
Greb’s research triumph is significant because it expands the definition of aromatic compounds to include species that are purely metallic. Utilizing elemental bismuth, the research team successfully isolated a ring made exclusively of bismuth atoms. This daring exploration into metal-based aromaticity underscores the versatility of elements beyond traditional organic frameworks. Acting as a precedent, it hints at the untapped potential that lies in exploring aromatic characteristics in other groups of metals. What differentiates this discovery is the methodology employed. By surrounding the positively charged metal ring with a negatively charged molecular cage, the team was able to protect the fragile structure from disintegration.
Supramolecular Stabilization: A Game Changer
The innovative approach of supramolecular stabilization opens new avenues for research and applications in the field of synthetic chemistry. This strategy not only aids in the isolation of fragile metal rings but could potentially herald a new era in the design of complex molecular assemblies. According to Greb, this technique of employing an outer molecular shell can be generalized to stabilize other positively charged species, thus broadening the possible constructs scientists could synthesize and study. This could fundamentally alter the landscape of chemical synthesis, allowing for greater creativity and ingenuity in the concoction of new materials.
Implications for Future Research
The implications of this discovery extend far beyond the confines of organic chemistry, suggesting a paradigm shift in our understanding of charge transport within metallic environments. The surprising effects observed through this research could pave the way for significant advancements in electronics and materials science. Such developments could ultimately lead to improved charge transport materials, opening up innovative applications in areas like energy storage and conversion, catalysis, and even nanotechnology.
As we stand on the threshold of a new chapter in chemistry, the isolation of metallic aromatic rings provokes reflection on the foundations of aromaticity itself. The notion that pure metal aromaticity can exist reshapes our understanding and encourages a re-evaluation of the chemical properties that dictate interactions between differing elements. Greb and his team are not just contributing to the expanding field of aromatic chemistry; they are setting the stage for a broader exploration of materials science that could redefine how we view and use metallic compounds in the future.

Leave a Reply