Antibiotic resistance is one of the most pressing public health dilemmas of our time, rendering many common infections perilous once again. As bacteria evolve and adapt, they often develop new defense mechanisms against the very drugs designed to eliminate them. This relentless game of cat and mouse between pathogens and scientists has sparked an urgent need for innovative solutions. In a groundbreaking study from the University of Illinois Chicago (UIC), researchers are trailblazing a path forward with an inventive class of antibiotics known as macrolones, which uniquely confront the bacterial enemy with a dual-targeting mechanism.
Understanding Macrolones and Their Mechanism
Macrolones represent a synthesis of two well-established categories of antibiotics: macrolides and fluoroquinolones. Macrolides, like erythromycin, traditionally inhibit protein synthesis by disrupting ribosomal functions—effectively shutting down the cell’s ability to produce the proteins necessary for survival. Meanwhile, fluoroquinolones, such as ciprofloxacin, specifically target DNA gyrase, an enzyme essential for bacterial DNA replication and stability.
This dual approach is revolutionary. By design, macrolones simultaneously hinder both protein production and corrupt DNA structure, presenting an almost impossible challenge for bacteria attempting to mount resistance. Research led by distinguished professor Alexander Mankin and his team highlights that a bacterium would need to acquire mutations to both targets at the same time—a feat deemed improbable given the sheer complexity of the genetic changes required.
An Unconventional Methodology
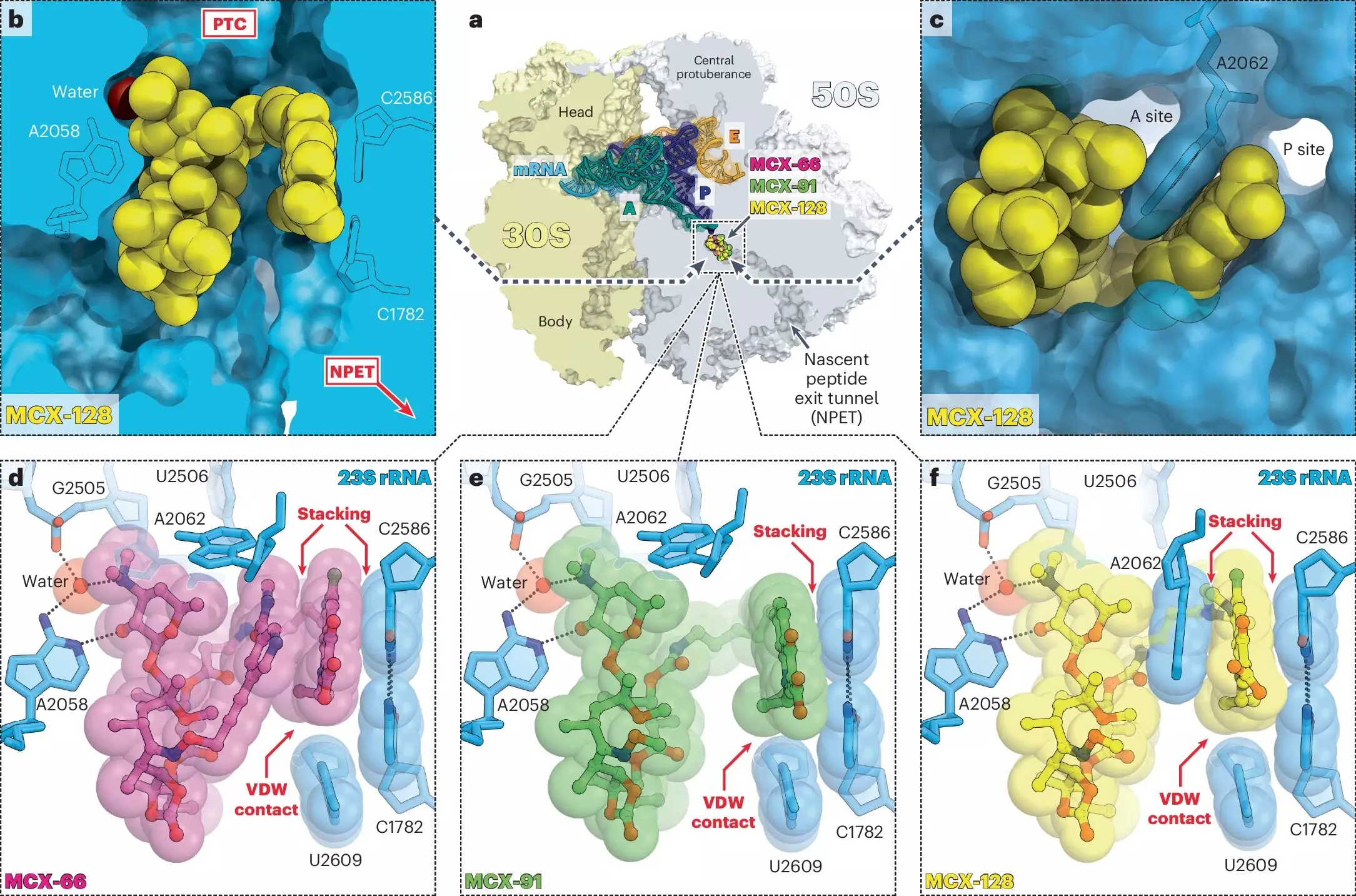
The study, recently published in *Nature Chemical Biology*, is rich with intellectual collaboration. Drawing from the diverse expertise pooled at UIC’s Molecular Biology Research Building, chemists and biologists alike have rallied to push the boundaries of antibiotic design. Researchers like Yury Polikanov, who specializes in structural biology, have provided critical insights into how these macrolones interact with the ribosome, leading to binding capabilities that surpass those of traditional macrolides, even in drug-resistant strains of bacteria.
These collective efforts underscore the importance of interdisciplinary research in curing complex health issues. When different scientific perspectives converge, it enhances innovative thinking and the potential to discover unconventional solutions.
Insights from Experimental Results
Through rigorous experimentation, findings indicate that while numerous macrolones showed promise for disrupting one specific target, only one compound emerged as the standout performer capable of impacting both cellular processes at low effective doses. This discovery aligns with Polikanov’s assertion: by targeting multiple cellular mechanisms simultaneously, the likelihood of bacteria evolving resistance diminishes dramatically. Simply put, this antibiotic is not just a tool; it’s a strategic weapon in our arsenal against bacterial infections.
Moreover, this research invites a paradigm shift in how we think about antibiotic development itself. It highlights the necessity of a more diversified approach in drug design—moving away from the singular target doctrine that has long dominated the field.
Future Directions for Antibiotic Research
As researchers like Mankin outline the way forward in refining the design of macrolones, this work lays the groundwork for future antibiotic therapies. There’s a clarion call for chemists to continue optimizing these drugs to maximize their dual-action efficiency against bacterial threats. The potential applications of this research extend beyond treating infections; they could also revolutionize surgical procedures and chemotherapy, where risks of infections are paramount.
The collaborative nature of this project acts as a beacon of light for future research endeavors, suggesting that the key to tackling antibiotics might lie not merely in isolated laboratory settings but in fostering environments where interdisciplinary teams can thrive and innovate.
The findings from UIC signify a substantial leap toward a more effective and resilient pharmaceutical landscape, igniting hope in a world desperately seeking answers to the escalating crisis of antibiotic resistance. Each successful advancement serves to underscore the notion that when tradition meets innovation, transformative breakthroughs are not only possible; they are imminent.

Leave a Reply