The complexities surrounding Alzheimer’s disease have long perplexed researchers and clinicians alike. Traditionally, amyloid fibrils—a stable configuration of protein aggregates found in the brains of Alzheimer’s patients—have been viewed primarily as harbingers of neurodegeneration. This view aligns with conventional wisdom that treating amyloid accumulation could offer a route to alleviating or even curing dementia. However, emerging research suggests a radical departure from this approach. A study led by Dr. Philip Kurian at Howard University’s Quantum Biology Laboratory proposes that quantum effects tied to these fibrils may actually play a vital role in protecting the brain from oxidative stress, rather than contributing to the disease’s progression.
In the context of Alzheimer’s research, amyloid fibrils are often regarded as the main adversaries to neuronal health. Yet, a significant number of individuals with high levels of amyloid in their brains never exhibit symptoms of dementia. This discrepancy raises pressing questions about the biological role of amyloid. Are these fibrils genuinely the villains they are portrayed to be, or could they represent a compensatory mechanism crafted by the brain in response to stress? The traditional methods aimed at targeting amyloid levels have yielded disappointing results in treatments. Against this backdrop, Kurian’s research compels a re-examination of how we view amyloid’s role in Alzheimer’s pathology.
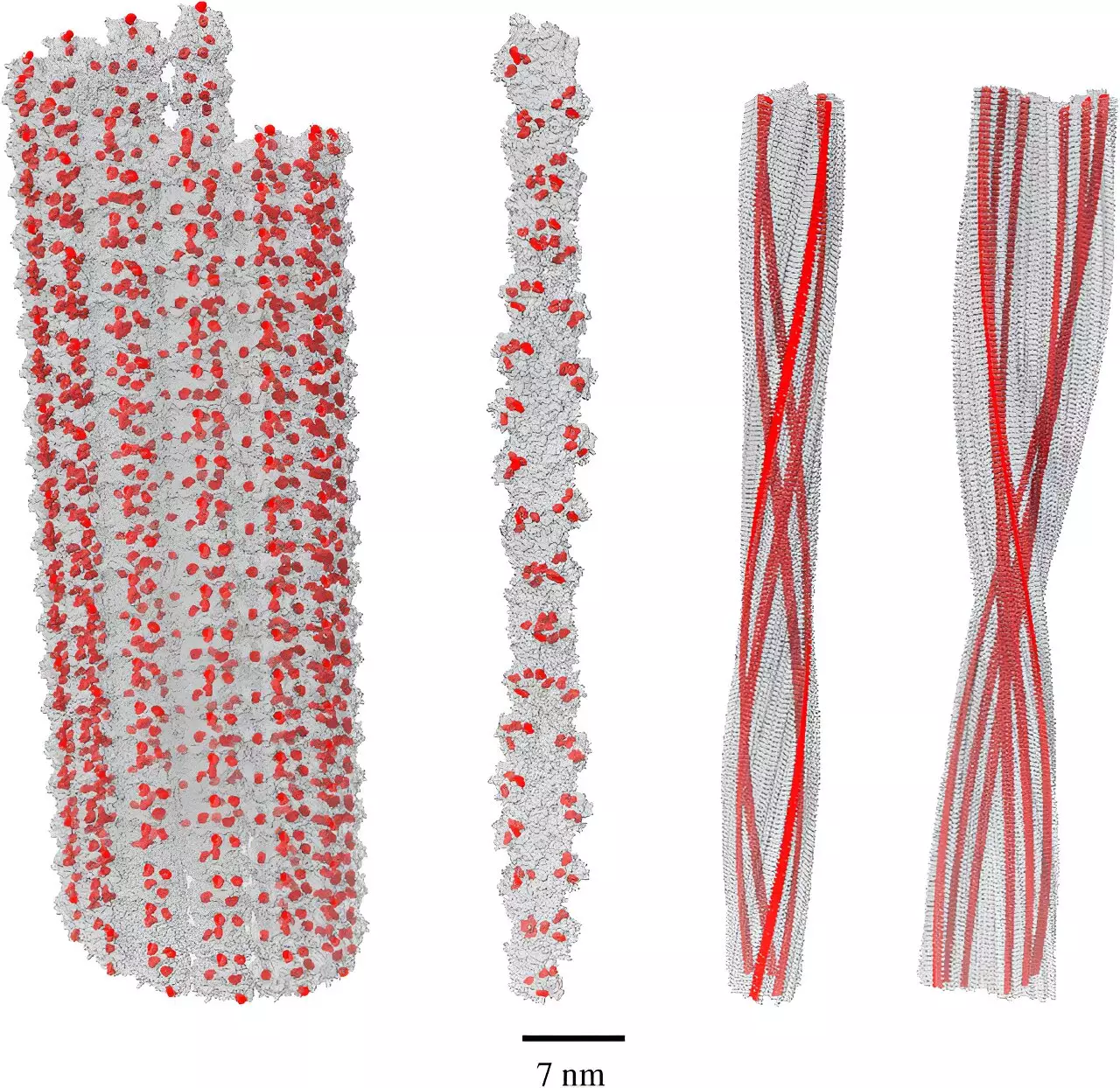
At the heart of Kurian’s findings is the phenomenon known as single-photon superradiance—an intricate quantum effect where networks of molecules, specifically amino acids like tryptophan, can efficiently process and transform harmful energy into safer forms. Previous work by Kurian’s team indicated that tryptophan networks could survive the tumult of biological systems while mitigating oxidative stress by absorbing high-energy UV photons. Now, their latest investigation reveals that amyloid fibrils possess a greater capacity for harnessing superradiance than previously understood. This revelation is critical as it suggests that amyloids may have intricate roles that extend beyond mere deterioration of neuronal function.
The study reveals that the structural arrangement of tryptophan molecules within amyloid fibrils is optimized for energy absorption and its subsequent release at a less harmful energy level. This photoprotective capability might imply that amyloid fibrils are not just passive aggregations of misfolded proteins, but reactive structures that help combat oxidative stress. The body’s struggle against oxidative stress involves the production of free radicals—unstable molecules that can cause significant cellular damage. The ability of amyloid fibrils to absorb and convert high-energy UV photons might, therefore, present a defensive mechanism rather than a pathological one.
Professor Lon Schneider, a noted authority in Alzheimer’s research, commended Kurian’s findings, noting their profound implications for the current treatment landscape. His assertion that amyloid aggregation could represent a protective response refutes a long-held premise that amyloid must be the target of therapeutic intervention. Instead, Kurian’s work highlights the importance of exploring alternative strategies that could harness the protective qualities of amyloid rather than merely diminishing its presence.
Additionally, Kurian called upon his colleagues in the fields of biology and neuroscience to broaden their interdisciplinary approach to research, advocating for the incorporation of quantum perspectives into traditional biological frameworks. The integration of quantum mechanics into these fields represents an avant-garde method to understand the underpinnings of life itself.
The next logical step in this exploration is to experimentally validate Kurian’s proposed mechanisms. As research continues to unveil the multifaceted roles of amyloid fibrils, the scientific community faces the exciting possibility of reframing Alzheimer’s disease—shifting from a quest to eliminate amyloids to one that embraces their complex roles in biological adaptation. Beyond the implications for Alzheimer’s, this work raises larger questions about the manner in which life forms interact with quantum phenomena—a burgeoning field that promises to deepen our understanding of biology.
As we stand at this pivotal intersection of biology and quantum mechanics, the journey ahead not only holds the keys to deciphering the tangled threads of neurodegeneration but also invites a more nuanced approach to life sciences, embracing complexity rather than seeking to simplify it. In doing so, we may not just illuminate the mysteries of Alzheimer’s but uncover the rich tapestry of interactions that characterize all living systems.

Leave a Reply