Fuel cells represent a pivotal innovation in the realm of sustainable energy generation, facilitating the conversion of chemical energy into electrical power through electrochemical reactions. Owing to their inherent design, fuel cells emit no harmful pollutants, making them a promising alternative to combustion-based energy sources. This technology holds immense potential for a diverse array of applications, including electric vehicles, portable energy devices, and various industrial machinery. However, the current landscape of fuel cell technology grapples with challenges, primarily due to the cost and availability of materials utilized in their construction.
A significant hindrance to the broader adoption of fuel cells has been the reliance on expensive noble metal catalysts and high-cost materials in their fabrication. These elements, while effective in catalyzing reactions necessary for fuel cell operation, impose substantial economic constraints. The need for an economically viable solution has led researchers to explore the potential of Anion-Exchange-Membrane Fuel Cells (AEMFCs), which promise a more affordable and sustainable alternative by utilizing abundant, less expensively sourced materials.
AEMFCs are emerging as a practical solution to the limitations observed in conventional fuel cell technologies. They utilize anion-exchange membranes to facilitate the transport of hydroxide ions, distinguishing them from their proton-exchange counterparts. This characteristic opens doors for the use of low-cost, non-precious metal catalysts, making fuel cells more accessible to various industries and applications. In recent years, research initiatives have intensified, aiming to develop and refine AEMFC technologies that prioritize both efficiency and cost-effectiveness.
Despite the promising outlook for AEMFCs, a notable challenge has surfaced regarding the stability and longevity of non-precious metal catalysts in fuel cells. Specifically, many of these catalysts, while potentially cost-effective, are prone to self-oxidation during operation. This process can lead to irreversible damage, thereby diminishing the performance and lifespan of the fuel cells. The need for an innovative solution to enhance the stability of these catalysts has become paramount in ongoing research.
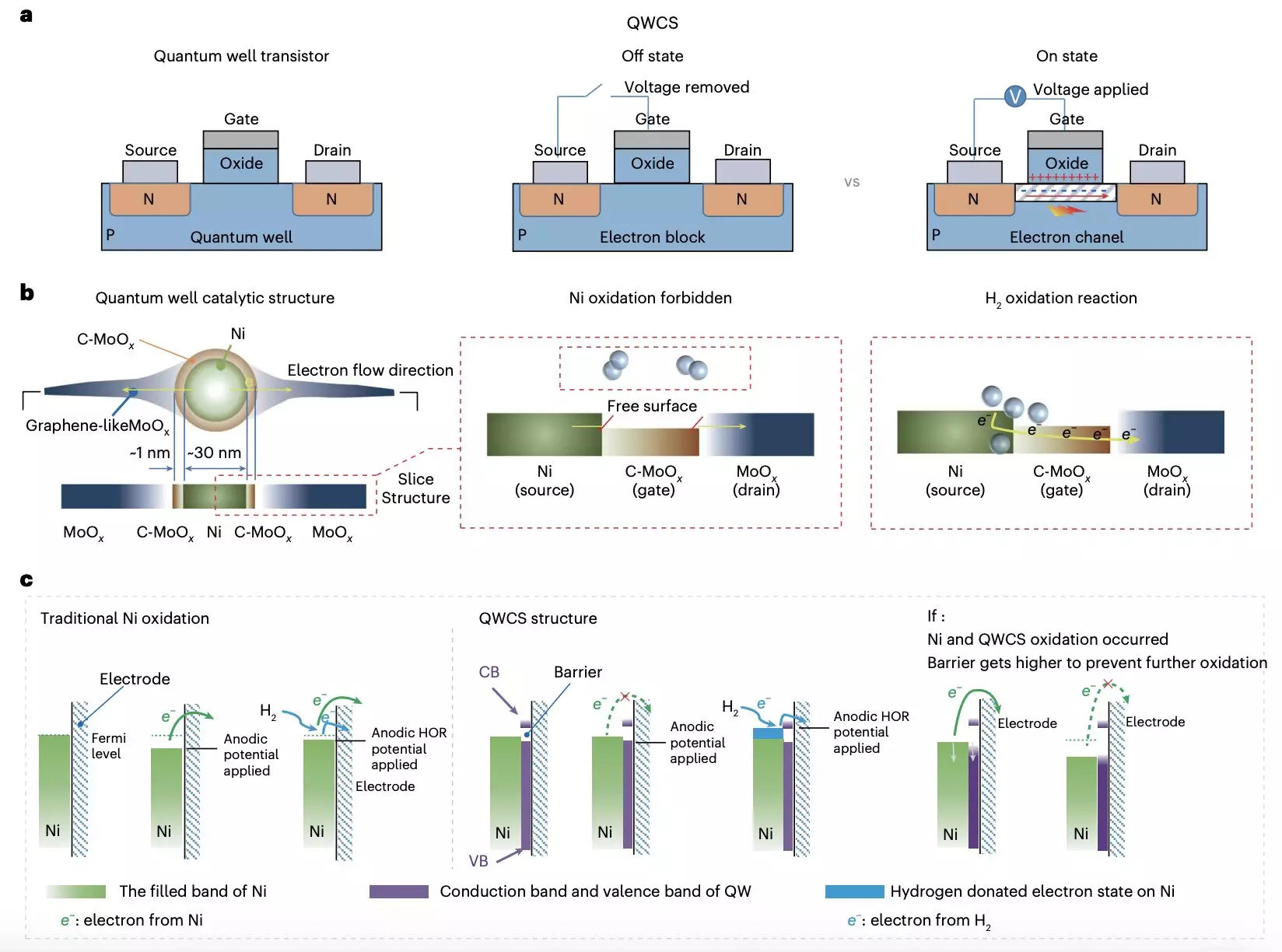
In a significant advancement, researchers from Chongqing University and Loughborough University have proposed a novel strategy to mitigate the oxidation issues faced by metallic nickel electrocatalysts in AEMFCs. Their breakthrough, detailed in a recent publication, describes the creation of a Quantum Well-like Catalytic Structure (QWCS) that employs quantum-confined nickel nanoparticles, effectively enhancing their longevity and performance. This innovative design not only stabilizes the metallic character of the nickel particles but also optimizes electron transfer during the hydrogen oxidation reaction, thus preventing the detrimental effects of oxidation.
The QWCS comprises a strategic arrangement of nickel nanoparticles atomically confined within a heterojunction that includes carbon-doped MoOx as a low-energy region, alongside amorphous MoOx representing a high-energy barrier. This sophisticated structure allows for selective electron transfer, a critical component in preserving the integrity of the catalyst under operational stress. Tests revealed that the new Ni@C-MoOx catalyst maintained impressive catalytic stability over extended periods, demonstrating robust performance even in harsh conditions.
The adaptability and resilience of the Ni@C-MoOx catalyst translated into remarkable outcomes for the AEMFCs designed for their implementation. The cells achieved an extraordinary specific power density of 486 mW mgNi^-1, with no observable decline in performance after multiple shutdown and restart cycles. Such results underscore the potential for QWCS-enabled AEMFCs to revolutionize the fuel cell market, propelling the viability of non-precious metal catalysts into practical use.
As we stand on the brink of a more environmentally friendly energy future, the advancements represented by the QWCS technology signal promising shifts in the design and application of fuel cells. By overcoming the barriers of cost and efficiency, the pursuit of widespread commercial adoption of AEMFCs seems increasingly attainable. Beyond its immediate applications, the research team’s innovative approach may also inspire further breakthroughs in catalyst development, leveraging principles of quantum confinement to expand the arsenal of materials amenable to sustainable energy solutions. As the quest for impactful technologies continues, the integration of these advanced catalytic structures could redefine the landscape of clean energy, fostering a more sustainable and economically viable future.

Leave a Reply