Cancer research is perpetually at the forefront of medical science, primarily because understanding the mechanisms of cancer cell proliferation is crucial for developing effective therapies. A critical area of focus lies in the role proteins play in the survival of cancer cells. Groundbreaking work at Scripps Research has ushered in a new era of cancer treatment paradigms by combining two sophisticated protein analysis methods. This research has led to the identification of over 300 small molecule-reactive cancer proteins, highlighting the importance of protein profiling in formulating targeted therapies that could revolutionize cancer treatment.
A significant challenge in cancer therapy has been the ability to accurately profile proteins associated with cancer cell survival and multiplication. Traditional approaches to protein profiling often fall short, failing to provide the granularity needed to identify all potential protein targets. This can lead to valuable targets being overlooked, stunting progress in drug development. The innovative team at Scripps has tackled this issue by employing a dual-method approach that intricately maps protein interactions, combining breadth with depth of analysis.
Co-senior author Benjamin Cravatt emphasizes the advantages of this novel methodology, stating that while one method yields overarching insight into protein interactions, the complementary method reveals the precise points of contact. This comprehensive landscape allows researchers to pinpoint specific sites on proteins that, when targeted by small molecule compounds, could impede cancer cell growth.
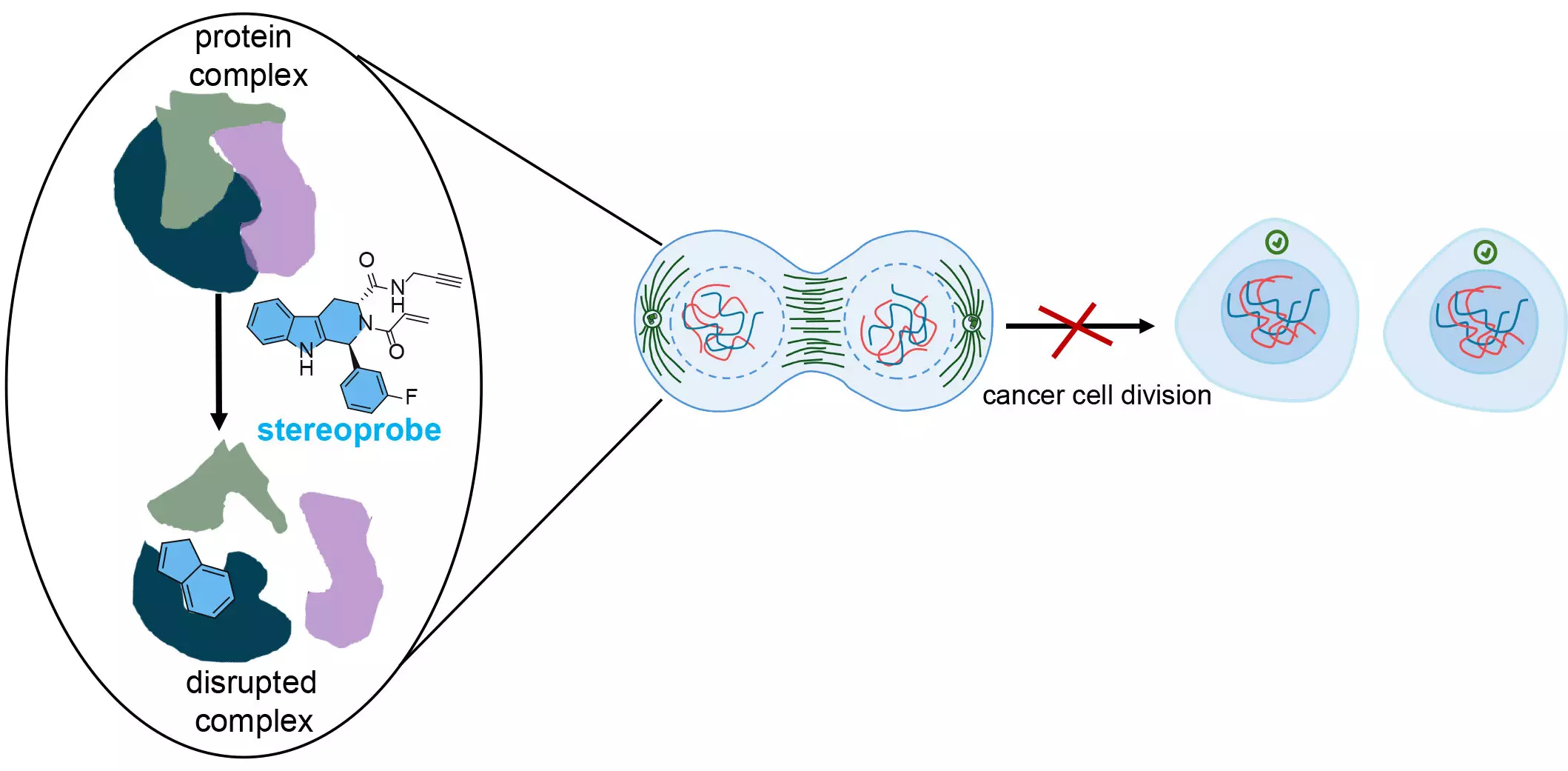
At the heart of this research is the use of stereoprobes—chemical compounds designed to selectively and permanently bind to specific proteins. These stereoprobes are engineered with unique chemical characteristics that are often underused in conventional drug discovery processes, providing a tactical advantage in identifying new protein targets. According to co-senior author Bruno Melillo, this deliberate design decision not only enhances discoverability but also lays the groundwork for advancements in human health.
The stereoprobes developed by the research team are electrophilic compounds that irreversibly bond with cysteine residues in proteins. Cysteine is prevalent in many proteins, including those tied to cancer, and its reactivity allows for significant disruption when targeted by chemicals. This capability to interfere with vital structural bonds within proteins is foundational to the therapeutic potential of these compounds.
The researchers utilized a technique known as protein-directed activity-based protein profiling (ABPP) to identify more than 300 proteins that reacted with their stereoprobes. However, this was only the beginning of their investigation. To achieve a granular understanding of the binding interactions, they implemented cysteine-directed ABPP, which illuminated the precise locations within the proteins where stereoprobes were binding. This methodological synergy enables researchers to “zoom in” on protein structures, akin to isolating a particular piece of a puzzle to assess its fit.
Each stereoprobe is characterized by its dual functionality: a binding component that recognizes protein pockets and an electrophilic component that facilitates the bond. When these stereoprobes effectively engage with their target protein, they can obstruct the protein’s ability to interact with its intended partners, thus initiating a cascade of events that slow cancer cell division. As first author Evert Njomen explains, this selective targeting disrupts cancer cells at crucial stages of their lifecycle, allowing the immune system to identify and eliminate the malfunctioning cells.
The implications of these findings are significant. The dual-method approach not only enhances the accuracy of protein profiling but also identifies critical regions within proteins that could be pivotal for drug development. Njomen notes that the research revealed a concerning amount of information lost when utilizing only one analytical platform, reiterating the necessity for comprehensive approaches in drug discovery.
Looking ahead, the research team aspires to extend their innovative techniques beyond oncology to explore stereoprobe applications in other diseases. Njomen expressed a desire to develop new stereoprobe libraries to investigate proteins implicated in various inflammation-linked disorders, opening up possibilities for treatment across multiple health issues.
Cancer treatment is on the brink of a transformative shift, thanks to the innovative strategies being developed. As researchers continue to refine and expand on these methodologies, the dream of developing targeted therapies that spare healthy cells while effectively combating cancer might soon become a reality, showcasing the profound impact of scientific collaboration and ingenuity on public health.

Leave a Reply