Clathrate hydrates are intricate water structures that encapsulate foreign molecules within a host water-molecule shell. These structures consist of water molecules, each comprising two hydrogen atoms attached to an oxygen atom, collectively forming networks that can bind weakly to each other and other molecules. The frameworks of clathrate hydrates are known as Frank-Kasper (FK) phases due to their close-packed tetrahedral geometric arrangement.
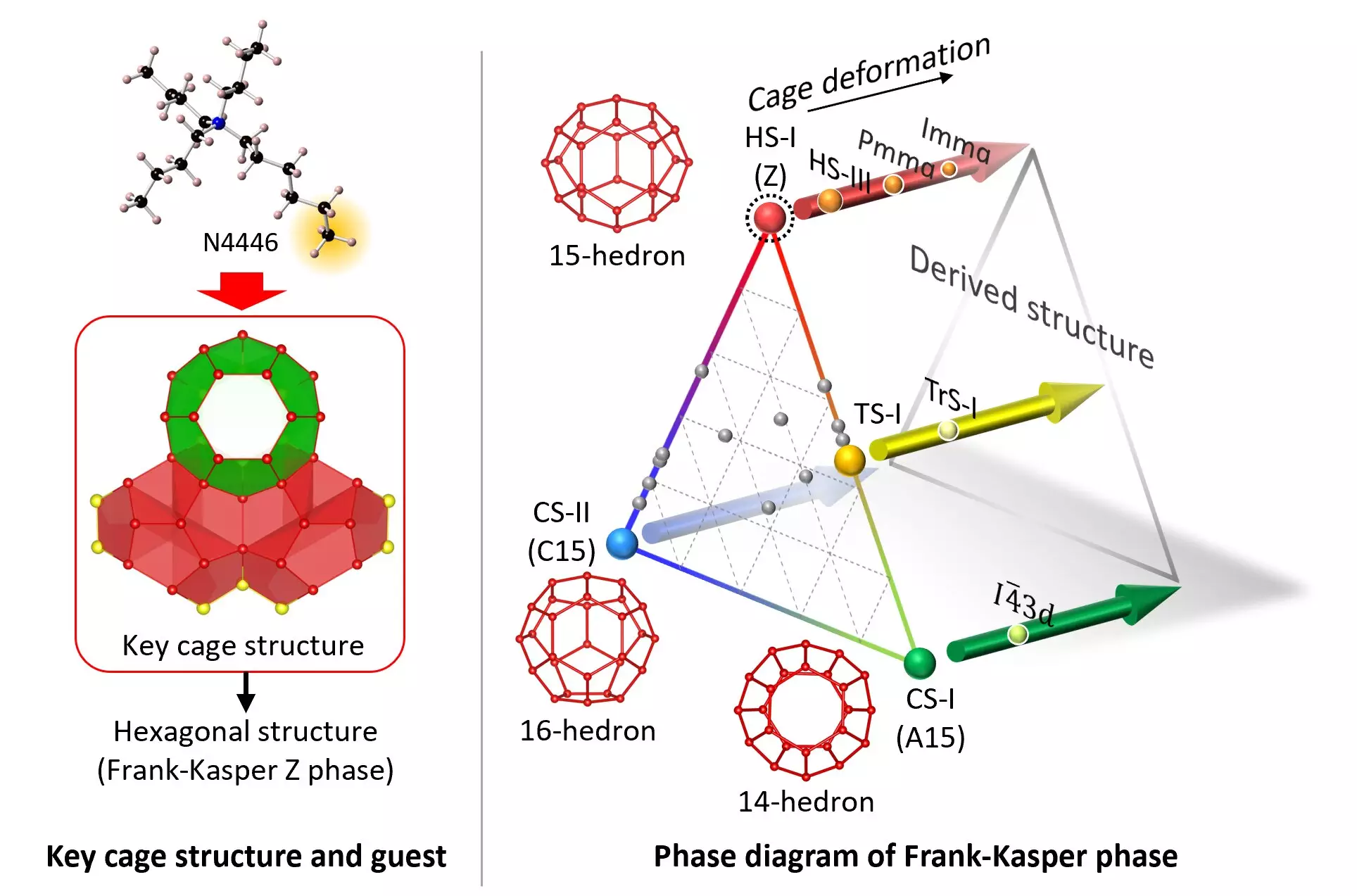
One notable clathrate hydrate phase, known as the HS-I structure, presents hexagonal crystals and had proven to be difficult to synthesize in a stable form. Previous attempts yielded a metastable variant of the HS-I structure, prompting a team of chemical engineers and crystallographers from Yokohama National University and the National Institute of Advanced Industrial and Science Technology (AIST) in Japan to explore the stabilization of this elusive clathrate hydrate.
The research team successfully created a stable form of the HS-I clathrate hydrate by precisely adjusting the guest molecule, tri-n-butyl n-hexylammonium chloride (N4446Cl). Through meticulous tuning of the guest molecule, specifically focusing on the n-hexyl chain, the team achieved the stabilization of the true pentakaidecahedron water-molecule cage essential for the HS-I structure. This breakthrough paves the way for the generation of a combination of mixed FK phases tailored for various applications.
Implications for Future Technologies
The ability to synthesize stable HS-I clathrate hydrate under ambient temperature and pressure conditions marks a significant advancement in material science. Previous research efforts predominantly aimed at creating new water structures under extreme conditions, such as ultrahigh pressure or ultracold temperatures. This recent achievement underscores the potential of leveraging physicochemical properties of diverse water lattices in ecological research and streamlining material development processes.
Broader Applications and Future Prospects
The synthesis of stable HS-I clathrate hydrate is poised to have a far-reaching impact across various fields, offering opportunities for enhancing storage and transportation technologies for natural gas, synthetic fuels, and carbon dioxide separation and recovery processes. Furthermore, the discovery of the final primitive structure of clathrate hydrates sets the stage for continued exploration in material science, with the potential for developing new materials incorporating mixed phases.
The successful stabilization of the HS-I clathrate hydrate represents a significant milestone in material science research. By unlocking the true structure of this complex water lattice, researchers have opened doors to a wide range of applications, from energy storage to environmental remediation. This groundbreaking achievement underscores the importance of collaboration among interdisciplinary teams in advancing scientific knowledge and pushing the boundaries of materials research.

Leave a Reply