Organic redox-active molecules (ORAMs) have emerged as a promising area of research in the quest for sustainable energy storage solutions. Their inherent diversity and abundance make them particularly attractive for applications in aqueous organic flow batteries (AOFBs), which are gaining traction in renewable energy storage paradigms. The unique properties of ORAMs suggest they could provide a more cost-effective alternative to traditional energy storage materials. However, key technical challenges must be addressed to realize their full potential, particularly their stability during the charge and discharge cycles.
One of the significant hurdles facing ORAMs is their stability during operation. When exposed to air, many ORAMs undergo side reactions that can deactivate their redox activity, ultimately leading to irreversible capacity loss in batteries. This instability is further exacerbated when these molecules are utilized without inert gas protections, rendering them less viable for practical applications. Hence, enhancing air stability has become a focal point of research efforts aimed at improving the efficiency and longevity of AOFBs.
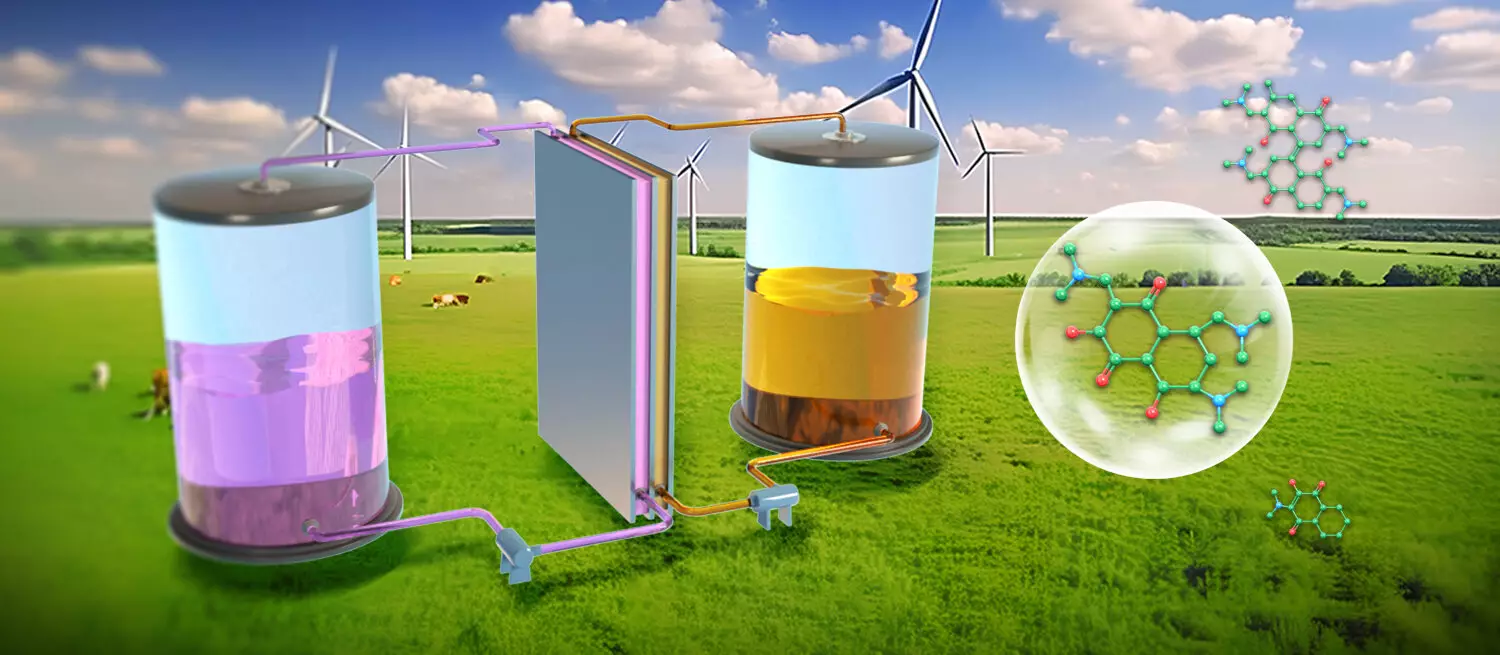
A notable advancement in addressing these challenges has been made by a research team from the Dalian Institute of Chemical Physics (DICP) in China. Led by Professors Li Xianfeng and Zhang Changkun, the team has engineered novel naphthalene derivatives that integrate active hydroxyls and dimethylamine scaffolds. These newly developed catholytes not only display remarkable air stability but also maintain effective redox activity in aqueous environments.
The research, published in *Nature Sustainability*, reveals that the synthesized naphthalene derivatives can undergo long-term cycling in exposed atmospheric conditions without significant degradation. This study emphasizes the systemic innovation of using a scalable synthesis approach that merges chemical and electrochemical techniques, making the purification process both simpler and more economical.
In their experiments, the team demonstrated that their naphthalene-based AOFBs could maintain stable cycling performance for up to 850 cycles, equivalent to around 40 days of operation, achieving a capacity of 50 Ah L-1. Even more impressively, with air continuously flowing through the catholyte, the battery sustained 600 cycles, approximately 22 days, without any loss in capacity or efficiency. These results affirm the exceptional air stability of the naphthalene derivatives and underscore their potential for real-world applications.
Moreover, the researchers successfully upscaled the preparation of these derivatives to a kilogram scale, producing about 5 kg per batch, which is promising for industrial applications. Pilot-scale battery stacks employing the naphthalene derivatives yielded an impressive average system capacity of about 330 Ah, with cycling stability maintained over 270 cycles and a capacity retention rate of 99.95% per cycle.
The breakthroughs by Professors Li and Zhang in developing air-stable ORAMs mark a significant stride toward advancing sustainable electrochemical energy storage technologies. As we confront the growing demands for energy storage solutions in a world increasingly reliant on renewable energy, these innovations may pave the way for more efficient, economical, and environmentally friendly systems. Given the outstanding performance metrics achieved, further research and development in this field hold the promise of transforming energy storage approaches in the years to come.

Leave a Reply