The synthesis of trisubstituted Z-alkenes poses a complex challenge in the field of organic chemistry, due to the inherent instability of these isomers compared to their E counterparts. These compounds are crucial in biological systems and are integral to the synthesis of various active pharmaceutical ingredients. Their role as substrates in stereospecific reactions catalyzes the formation of sp3-hybridized structures, which are pivotal in the development of new drugs. Therefore, methods that can selectively produce Z-isomers are highly sought after. Previous methods have neglected a critical aspect of Z-alkenes’ synthesis, primarily due to a thermodynamic bias leading to low yields and a lack of efficient catalytic processes.
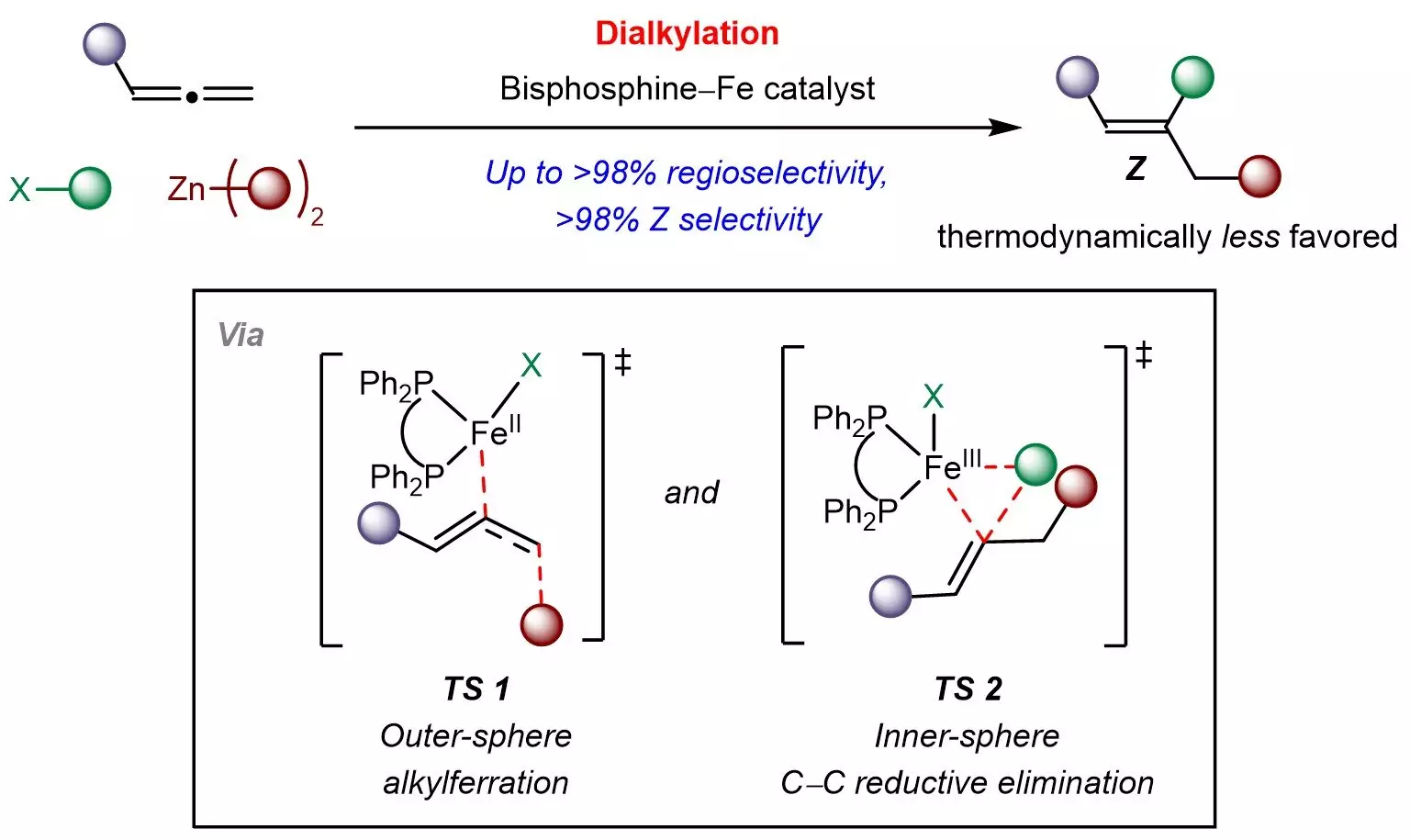
In a notable advancement, chemists from the National University of Singapore (NUS) led by Associate Professor Koh Ming Joo have introduced an innovative iron-catalyzed strategy for overcoming these obstacles. This method utilizes a cost-effective bisphosphine–iron catalyst to facilitate the integration of allenes with simpler organic compounds like sp3-hybridized organohalides and organozinc reagents. This multicomponent reaction not only adds diverse aliphatic groups to the allene but also offers precise control over both site and Z-selectivity.
The use of iron, a non-toxic and widely available transition metal, further underscores the economic and ecological benefits of this approach. Enabling access to these complex compounds in a sustainable manner showcases a significant stride towards greener chemistry. Thus, the methodological improvements offered by this iron-based catalyst have the potential to revolutionize the way Z-alkenes are synthesized, making them more accessible for research and industrial applications.
The practical implications of this research extend to the pharmaceutical industry, particularly highlighted by the successful application of this method in the synthesis of a glucosylceramide synthase inhibitor that specifically requires Z-configuration for its activity. This directs attention to the capability of this approach not only to produce challenging compounds but also to streamline processes involved in drug discovery. Such advancements enable chemists to further explore the bioactivity of various Z-alkenes, providing insights that could lead to enhanced therapeutic interventions.
The findings underscore a breakthrough in our understanding of reaction mechanisms, pointing to a unique pathway involving outer-sphere radical-mediated alkylferration, which is followed by inner-sphere carbon-carbon bond formation. According to Professor Koh, these insights pave the way for designing new kinetically controlled reactions involving allenes and other pi-systems.
Moreover, the NUS team’s explorations are set to include the development of additional multicomponent reactions aimed at converting abundant raw materials into higher-value chemical products, thus positioning this research project at the forefront of sustainable chemistry. Such initiatives are vital in addressing not only the chemical industry’s demands but also the global push for environmentally friendly production processes. Through ongoing collaboration and innovative research, the future looks promising for the utilization of these groundbreaking synthetic methods across various industries.

Leave a Reply