The contamination of water bodies with heavy metals represents one of the most pressing environmental challenges faced globally. These pollutants—such as lead and cadmium—pose significant threats not only to human health but also to aquatic ecosystems. The development of effective and sustainable solutions for heavy metal removal is crucial as traditional methods often prove to be costly and inefficient. Recent research has unveiled promising new approaches involving sugar-like polymers derived from plants that could revolutionize water purification efforts.
Heavy metals in drinking water can lead to various health problems, including neurological damage, kidney disease, and other serious conditions. Often, conventional methods of water treatment—like filtration—can be energy-intensive and create further environmental concerns due to the need for frequent replacement of clogging membranes. This necessitates the development of more sustainable and efficient solutions. A promising area of interest is the use of naturally occurring materials, particularly those derived from plants, to tackle this pressing issue.
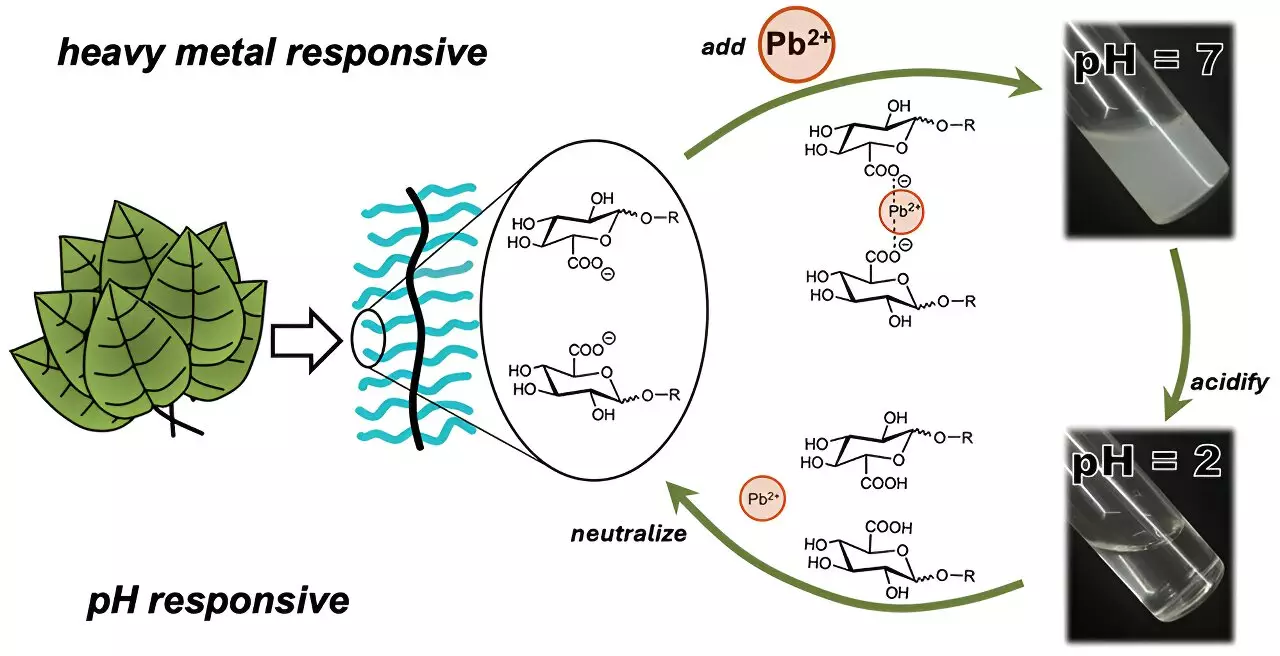
Research over the years has indicated that plants possess inherent abilities to detoxify their environments. Their cellular structure is fortified with barriers consisting of polysaccharides, complex carbohydrates made of sugar molecules that play a significant role in metal ion binding. Previous studies have examined the potential of polysaccharides extracted from plants like okra and aloe to remove microplastics from wastewater, highlighting the relevance of natural substances in water purification technologies.
In a significant innovation, researchers led by Cassandra Callmann at the University of Texas at Austin have developed a novel sugar-derived polymer that efficiently captures heavy metals from contaminated water. This polymer possesses a unique structure that combines a water-insoluble backbone with various water-soluble carbohydrate “charms,” akin to beads on a string, which are specifically designed to target and bind metals.
Through meticulous experimentation, the team identified that a specific carbohydrate modification featuring a carboxylic acid group optimized the polymer’s capacity to attract polar metal ions, particularly cadmium. In preliminary tests using water spiked with ionic cadmium, the polymer swiftly formed visible clumps within just three minutes, simplifying the removal process through filtration methods.
The allure of this new polymer lies not only in its efficiency but also in its recyclability. After several cycles of binding, clumping, and redissolving—achieved by adjusting the water’s acidity—the polymer retained its heavy metal capturing proficiency, establishing a foundation for developing reusable materials for water purification.
Following laboratory success, the team extended their investigation to real-world scenarios by testing their carbohydrate-rich polymer on Colorado River water, which had been spiked with ionic cadmium and lead. The river water, rich in other ions like calcium and magnesium, presented a true challenge for selective metal removal. However, the results were striking: the polymer successfully captured 20% of the cadmium and a remarkable 45% of the lead over a 24-hour observation period, with minimal interference from other metal ions.
These findings underscore the polymer’s potential to be an innovative solution in the burgeoning field of water purification technologies. As the need for practical, scalable methods to address water contamination continues to grow, this research paves the way for future advancements in the development of efficient, sustainable materials for heavy metal removal.
The research by Callmann and her team is a compelling indication of how biomimetic approaches can lead to significant breakthroughs in environmental sciences. By harnessing the natural properties of plant-derived materials, they have designed a polymer that not only effectively traps heavy metals but does so in a way that promotes sustainability and recyclability. As the world grapples with water quality issues, innovations like these serve as critical components of a more comprehensive strategy to ensure safe drinking water and protect aquatic ecosystems from the detrimental effects of pollution. Future research and development efforts will be essential in translating these laboratory results into widespread practical applications, ultimately contributing to cleaner water for all.

Leave a Reply