Chemical reactions are complex processes that often involve the formation of intermediate compounds before the final products are obtained. Understanding these intermediate steps is crucial for advancements in various fields, including energy generation, catalysis, and medicine. However, visualizing these short-lived intermediates at the atomic level has proven to be quite challenging. One innovative method that shows promise in this area is time-resolved serial femtosecond crystallography (TR-SFX).
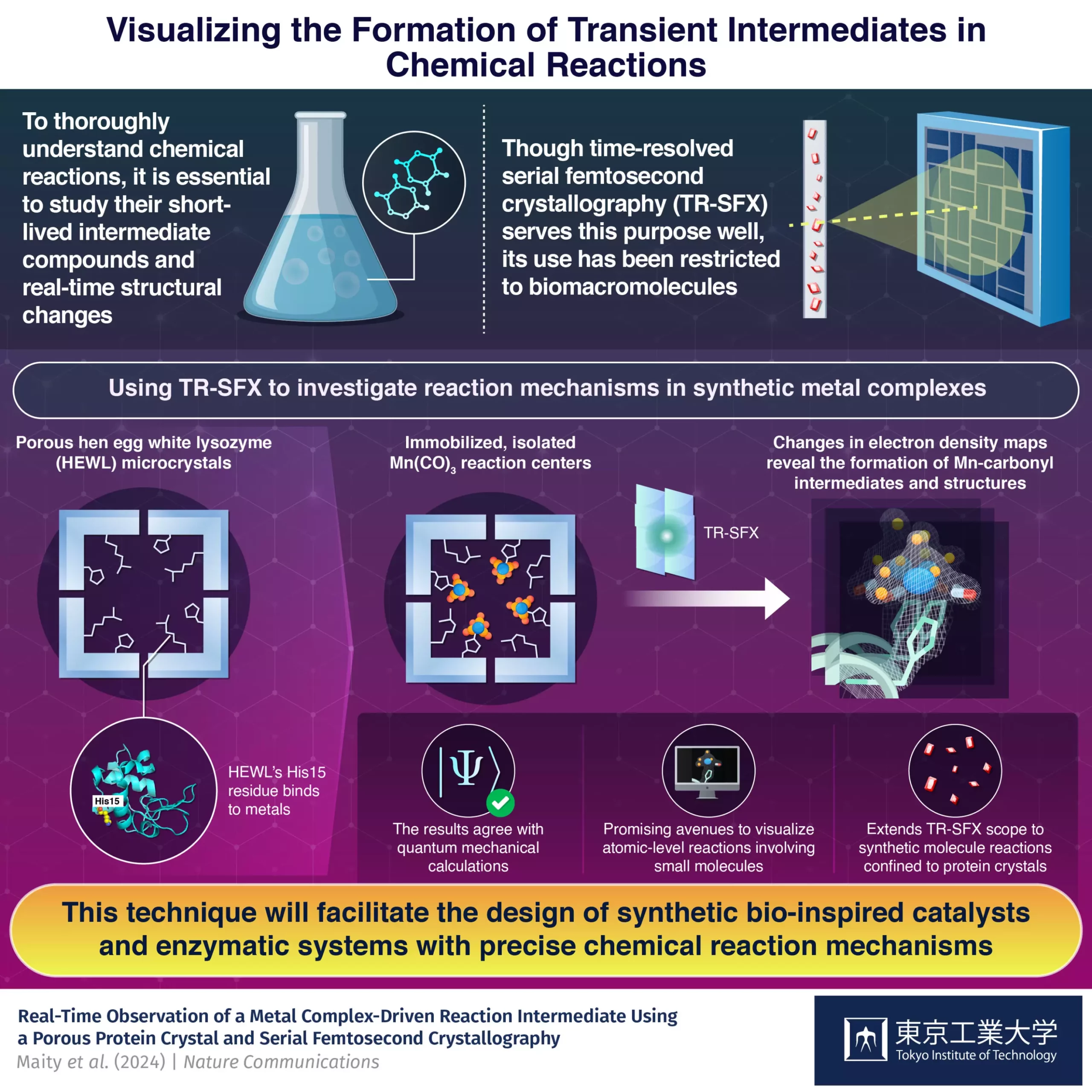
While TR-SFX has been primarily used with biomacromolecules, a research team from Japan led by Professor Takafumi Ueno sought to extend its applications to synthetic compounds. The team successfully captured the dynamics of carbon monoxide (CO) release from Mn(CO)3, overcoming challenges associated with the use of small molecules in TR-SFX. By developing an innovative strategy based around hen egg-white lysozyme (HEWL) crystals, they were able to create a nanoporous structure suitable for studying reactions in synthetic compounds.
The researchers immobilized a light-sensitive Mn(CO)3-containing compound inside HEWL crystals, providing a controlled environment for studying the CO release reactions. By shooting light pulses at the crystals at carefully controlled time intervals relative to the TR-SFX electron laser pulses, they were able to analyze changes in electron density maps and capture the reaction dynamics within the protein crystals.
The results of the study were in excellent agreement with quantum mechanical calculations, validating the proposed strategy. The researchers demonstrated that by using protein crystals as a matrix, the reactions of synthetic metal complexes can be studied in detail, including the determination of intermediate structures. This breakthrough holds the potential to advance the design of artificial metalloenzymes with precise mechanisms, enabling the development of new drugs, catalysts, and enzymatic systems involving non-biological components.
The integration of small synthetic molecules inside protein crystals combined with time-resolved serial femtosecond crystallography offers a groundbreaking approach to studying reaction dynamics at the atomic level. This innovative strategy opens up new possibilities for the design and development of drugs, catalysts, and functional materials, ultimately contributing to advancements in chemical research and applications across various scientific disciplines.

Leave a Reply