The pressing issue of climate change has garnered significant attention in recent years, with carbon dioxide (CO2) emissions being recognized as a primary contributor to global warming. As industries and governments seek effective solutions to combat this phenomenon, innovative approaches in material science are emerging. Among these, the carbonation of cement-based materials presents a promising avenue for capturing and transforming CO2 into stable mineral forms. This process not only offers potential to mitigate greenhouse gas emissions but also opens up new horizons for sustainable construction materials.
Carbonation, in the context of cement paste, involves a series of intricate chemical reactions that transform CO2 into carbonate minerals. When CO2 dissolves in water, it engages in complex interactions with calcium silicate hydrates (C–S–H), the byproducts of cement hydration. During this process, the dissolved CO2 gives rise to carbonate ions, which then react with calcium ions to precipitate calcium carbonate. Despite extensive research on this phenomenon, a comprehensive understanding of the carbonation mechanism remains elusive. This ambiguity is largely attributed to the reactive and unstable nature of cement paste compounds, which complicates the precise characterization of the carbonation process.
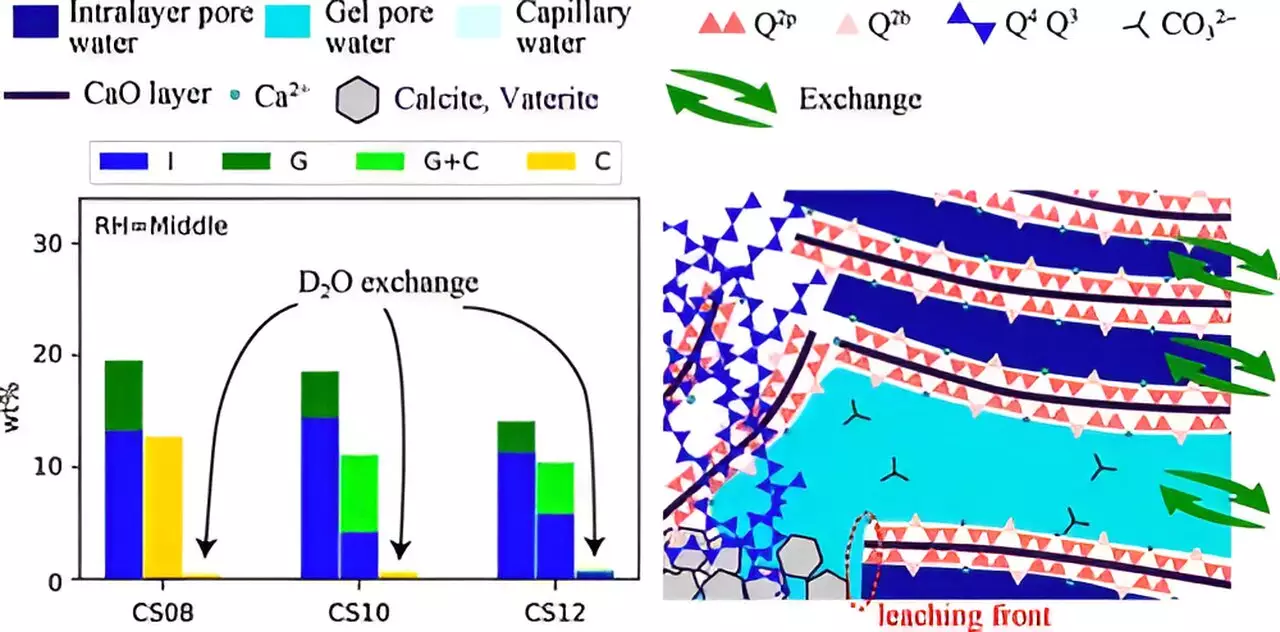
The parameters influencing this reaction are myriad, including relative humidity (RH), calcium/silicate (Ca/Si) ratio, and the saturation levels of water within the C–S–H matrix. Notably, the behavior of water molecules and ion transport through the nanoscale pores of C–S–H, which contain gel-pore water, remains a critical area of investigation. Understanding these dynamics is essential for optimizing the rate and efficiency of carbonation reactions.
Recent advancements in research methodologies have shed new light on the carbonation mechanisms at play. A notable study led by Associate Professor Takahiro Ohkubo from Chiba University and his team employed advanced techniques to explore how various conditions affect carbonation. By utilizing 29Si nuclear magnetic resonance (NMR) and 1H NMR relaxometry, the researchers provided valuable insights into the processes governing water transport and structural changes during carbonation.
Accelerated carbonation experiments were conducted under controlled conditions using a significantly elevated concentration of CO2, simulating the conditions that can occur over extended periods in natural settings. This approach helped bridge the gap between the slow natural carbonation processes and the faster results achievable in laboratory environments. The experiments varied with respect to RH conditions and the Ca/Si ratios, allowing for a nuanced understanding of how these factors interact.
The findings from this research distinctly illustrated that the carbonation reaction leads to notable structural changes within the C–S–H network. Not only does the chain structure of C–S–H undergo collapse, but the pore size within these materials is also altered significantly. The interplay between RH levels and the Ca/Si ratio emerged as a critical influence on the carbonation process. For instance, lower RH combined with a high Ca/Si ratio contributed to smaller pore structures, which, in turn, impeded the leaching of calcium ions and water, ultimately resulting in less effective carbonation.
Importantly, these observations underscore the necessity to consider both structural modifications and mass transfer dynamics together rather than isolating them. This comprehensive perspective is crucial for developing more efficient cement-based materials capable of higher CO2 capture.
A Broader Impact on Sustainable Development
The implications of Associate Professor Ohkubo’s team’s findings extend beyond the realm of cement chemistry; they hold significant promise for the future of sustainable construction practices. The research points towards the potential development of innovative building materials designed to absorb substantial quantities of atmospheric CO2. Moreover, understanding carbonation processes can inform broader environmental applications, particularly in the context of organic matter, which undergoes similar transformations.
This study not only advances the scientific understanding of carbonation in cement-based materials but also highlights a strategic pathway toward addressing global CO2 emissions. As the world grapples with climate change, enhancing the efficiency of materials that sequester CO2 can play a vital role in a more sustainable future. The pursuit of greener solutions within the construction industry is more than a mere technical endeavor; it is a necessary step in our collective responsibility toward the planet.

Leave a Reply