The quest for sustainable and renewable energy sources has led researchers to explore various methods for producing hydrogen, a clean fuel alternative that could play a significant role in reducing our reliance on fossil fuels. One innovation is the electrolytic splitting of water into hydrogen and oxygen, a process that can be enhanced with the use of photoelectrochemical cells (PEC cells). By harnessing solar energy, these cells utilize photoelectrodes to generate the electrical voltage necessary for electrolysis. Recent studies have highlighted a novel approach to improving the efficiency of these PEC cells—operating them under elevated pressure conditions.
Understanding Photoelectrochemical Cells
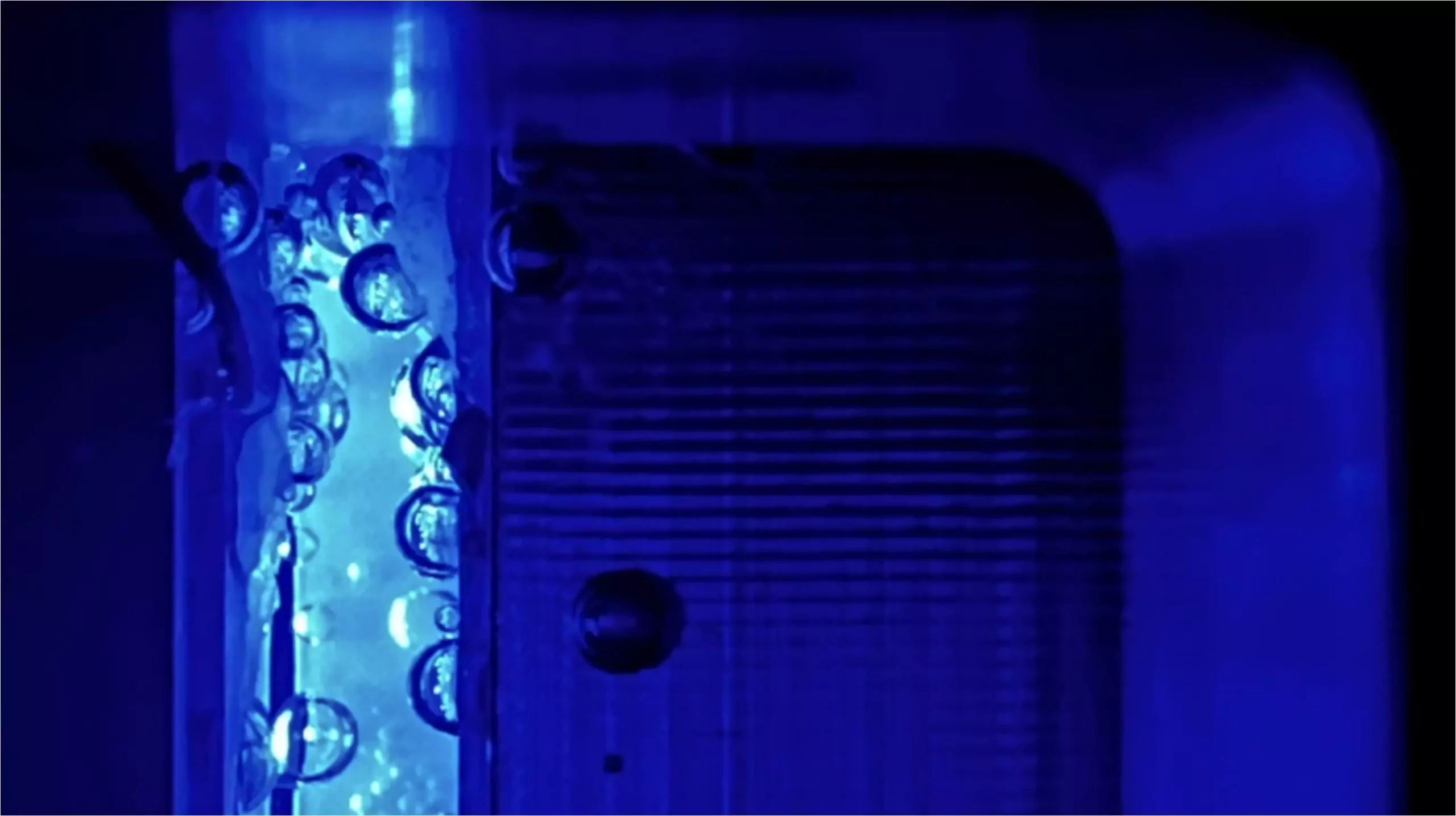
PEC cells mimic the natural process of photosynthesis found in plants, particularly the function of Photosystem II, which splits water using sunlight. Instead of the complex organic structures found in nature, PEC cells employ synthetic inorganic photoelectrodes to achieve electrical conversion. Early advancements in these systems indicated substantial energy conversion efficiencies, with some devices achieving rates as high as 19%. However, this efficiency comes with challenges, particularly related to the formation of gas bubbles, which interfere with the optimal illumination of the electrode and can prevent proper contact between the electrolyte and the electrode surface.
As PEC cells operate, hydrogen and oxygen gases are generated, which coalesce into bubbles on the electrode surface. These bubbles can scatter light, thereby reducing the voltage generated from the sunlight and deteriorating the overall efficiency of the system. Additionally, they may obstruct the interactions necessary for the ongoing electrochemical reactions, leading to performance setbacks. Traditionally, PEC cell operations have been conducted at atmospheric pressure (1 bar), where these efficiency losses are a significant concern.
To counteract the drawbacks of bubble formation, recent research from the Institute for Solar Fuels at HZB proposed a solution: increasing the operational pressure within PEC cells. This approach seeks to minimize bubble size and improve electrode performance, thus enhancing the overall efficiency of the photoelectrochemical process.
The research led by Dr. Feng Liang involved rigorous experimentation with PEC cells pressurized between 1 and 10 bar. The team developed a multiphysics model of the PEC process to analyze how elevated pressure impacts gas bubble size and behavior during electrolysis. Their findings were promising; increasing the operational pressure to around 8 bar resulted in a significant reduction in energy loss—up to 50%. This translated into an overall efficiency improvement of approximately 5-10%, a notable leap in the performance of hydrogen production methods.
By operating at elevated pressures, the study also demonstrated that light scattering caused by gas bubbles could be nearly eliminated, allowing for better light penetration and utilization. Additionally, the research identified a decrease in gas cross-over—specifically, the unintended transfer of oxygen to the counter electrode—enhancing the efficiency of the entire system.
While the results showed improvements at elevated pressures, the team observed that pushing pressures beyond 8 bar did not yield additional benefits. Therefore, they determined that the optimal pressure range for PEC electrolyzers lies between 6-8 bar. This balance maximizes efficiency while mitigating the challenges associated with bubble formation.
The implications of these findings extend beyond PEC cells to other electrochemical and photocatalytic applications. The multiphysics model developed by the HZB team presents a versatile framework for further research, suggesting that these efficiency gains could apply to various systems aiming for higher performance in hydrogen production.
The quest for efficient hydrogen production continues to evolve, bolstered by innovative approaches such as the use of elevated pressure in PEC cells. As researchers like Dr. Liang and Prof. Dr. Roel van de Krol delve deeper into optimizing these systems, the potential for enhanced energy conversion efficiencies not only supports advancements in renewable energy technologies but also brings us closer to a more sustainable energy future. By unlocking new strategies for overcoming the limitations of current techniques, such research holds the promise of significantly contributing to the global hydrogen economy.

Leave a Reply