For many years, the scientific comprehension of histones primarily revolved around complex multicellular organisms. These proteins play a crucial role in the packaging and organization of DNA, which is essential for its functionality and accessibility within a cell. However, recent research led by Samuel Schwab, a doctoral student at the Leiden Institute of Chemistry, reveals that single-celled organisms, specifically bacteria and archaea, also contain these proteins, and their diversity is astonishing. Schwab’s groundbreaking study, published in *Nature Communications*, posits that there are at least 17 distinct types of histones in these microorganisms, each boasting unique structures with potentially varied functions.
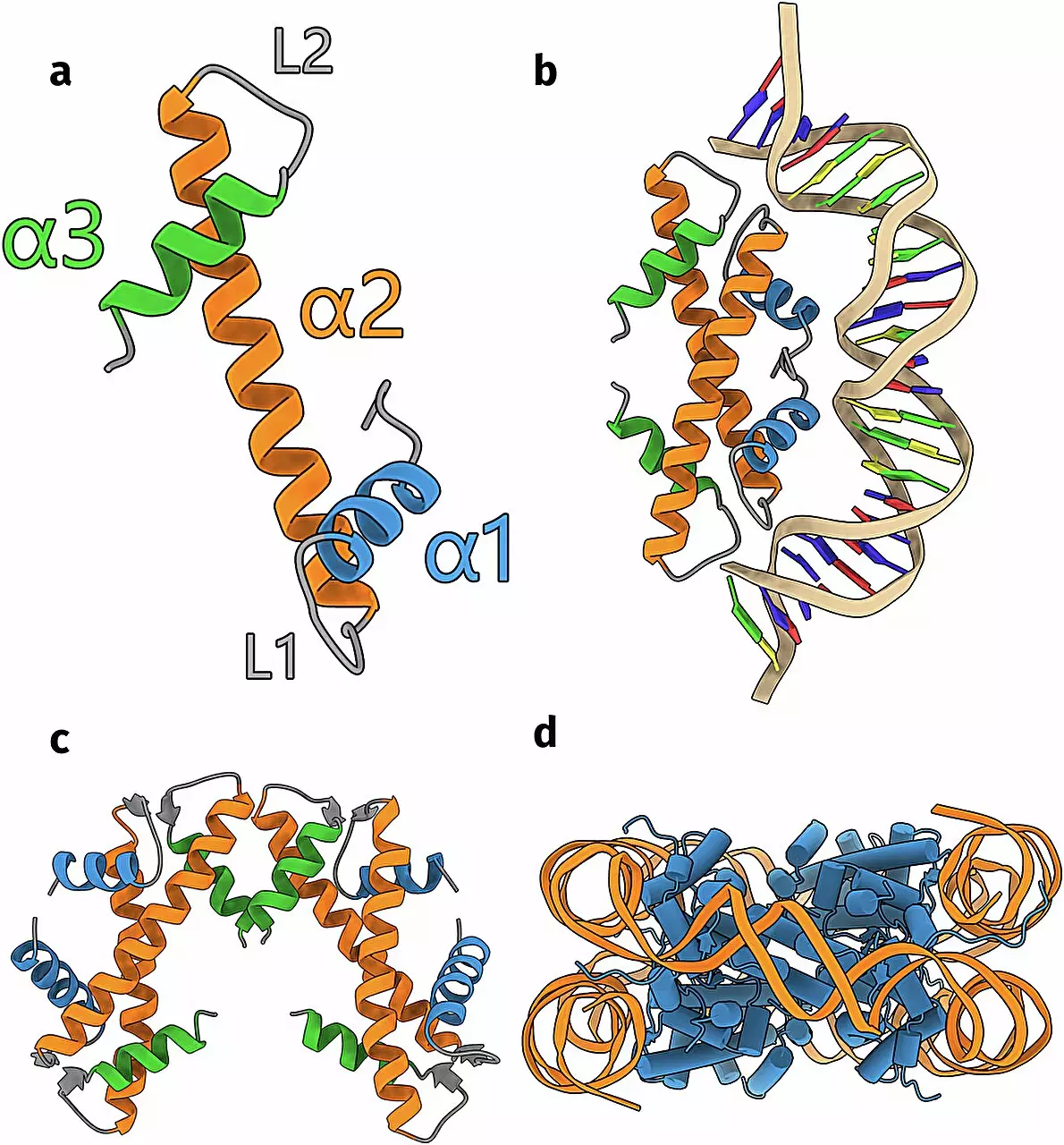
To understand the significance of Schwab’s findings, one must first comprehend the function of histones. Typically, histones encompass DNA to form complexes known as nucleosomes. This compaction is vital since DNA, in its uncoiled form, is far too voluminous to fit within a typical cell. It was long believed that only complex organisms possessed histones, a concept that was recently challenged when scientists identified these proteins in archaea and bacteria. Schwab’s research indicates that there may be a plethora of unrecognized histone forms that not only aid in DNA compaction but may serve other crucial functions within these microorganisms.
The revelation that histones can have multiple roles challenges the prevailing understanding and invites a reevaluation of how we conceptualize genetic material organization in less complex life forms. Schwab’s exploration into the diverse types of histones suggests a more dynamic interaction with DNA than previously acknowledged.
A cornerstone of Schwab’s research lies in the utilization of AlphaFold, a sophisticated AI algorithm designed for protein structure prediction. For over three years, this technology has enabled scientists to predict the structures of proteins by analyzing their corresponding DNA sequences—an otherwise laborious endeavor. Schwab collected comprehensive data, amassing around 6,000 DNA sequences suspected of encoding histones in archaea and bacteria. By employing AlphaFold in conjunction with the Leiden supercomputing facility, ALICE, he successfully elucidated the structures of these proteins.
The findings revealed a remarkable diversity among histones with some structures previously known but many that were entirely new to the scientific community. This triumph not only underscores the efficacy of AI in scientific research but also demonstrates that integrating technology into biological studies can yield substantial advancements in our understanding of life at a molecular level.
The validation of AlphaFold’s predictions constituted a significant aspect of Schwab’s work. The researchers physically examined one of the newly predicted histone structures, confirming it was nearly identical to what the AI had proposed. This cross-verification accentuates the synergy between computational methods and experimental biology. With these validated structures, the team ventured into hypothesizing how these proteins interact with DNA.
They discovered that besides the well-known spherical nucleosome formations that wrap DNA, some histones exhibited remarkable capabilities of folding or bridging DNA strands. This versatility hints at a more complex regulatory framework guiding genetic expression and DNA manipulation than current paradigms suggest.
In tandem with the many exciting discoveries represented in Schwab’s work, a particularly intriguing finding emerged regarding the interactions between certain histones and cellular membranes. Rather than merely influencing DNA structure, these histones demonstrated potential alternative roles, warranting further investigation. Schwab’s supervisor, Remus Dame, highlighted the need to delve deeper into these non-traditional histone functionalities, which could provide new insights regarding cellular organization and function.
This research lays the groundwork for an expansive understanding of DNA organization across various forms of life, illuminating the evolutionary pathways that have shaped how different organisms manage their genetic material. As Schwab aptly states, there’s an ocean of knowledge still waiting to be uncovered.
The exploration of histone diversity in archaea and bacteria signals a paradigm shift in genetics, prompting scientists to rethink the broader implications of protein structure within the microscopic realm. The convergence of advanced technology, rigorous research, and a new perspective on genetic organization promises to unveil much about the operating principles of life at its most fundamental level.

Leave a Reply