As the world grapples with escalating carbon emissions and climate change, innovative methods to repurpose carbon dioxide (CO2) into sustainable fuels are emerging as essential solutions. Notably, a groundbreaking artificial photosynthesis system developed at the University of Michigan has achieved the remarkable feat of efficiently combining carbon atoms into hydrocarbons. This system not only excels in performance but also opens the door to carbon-neutral fuel alternatives that could dramatically reduce our current reliance on fossil fuels.
The University of Michigan’s device excels in creating ethylene, a vital hydrocarbon that predominantly serves as a precursor for plastics. Traditional methods for producing ethylene are energy-intensive and involve the combustion of oil and gas, contributing significant CO2 emissions. In contrast, the artificial photosynthesis system demonstrates an efficiency and longevity unprecedented in current technologies. According to Zetian Mi, a professor in electrical and computer engineering, this innovation surpasses existing solar-driven CO2 reduction systems by a margin of five to six times, underscoring its potential ramifications on both sustainability and industrial production practices.
This sequential transformation of CO2 into hydrocarbons not only facilitates the conversion of waste gases into valuable products but also significantly curbs harmful emissions into the atmosphere. Given that ethylene is the world’s most produced organic compound, this system opens a pathway to fundamentally altering how plastics are manufactured.
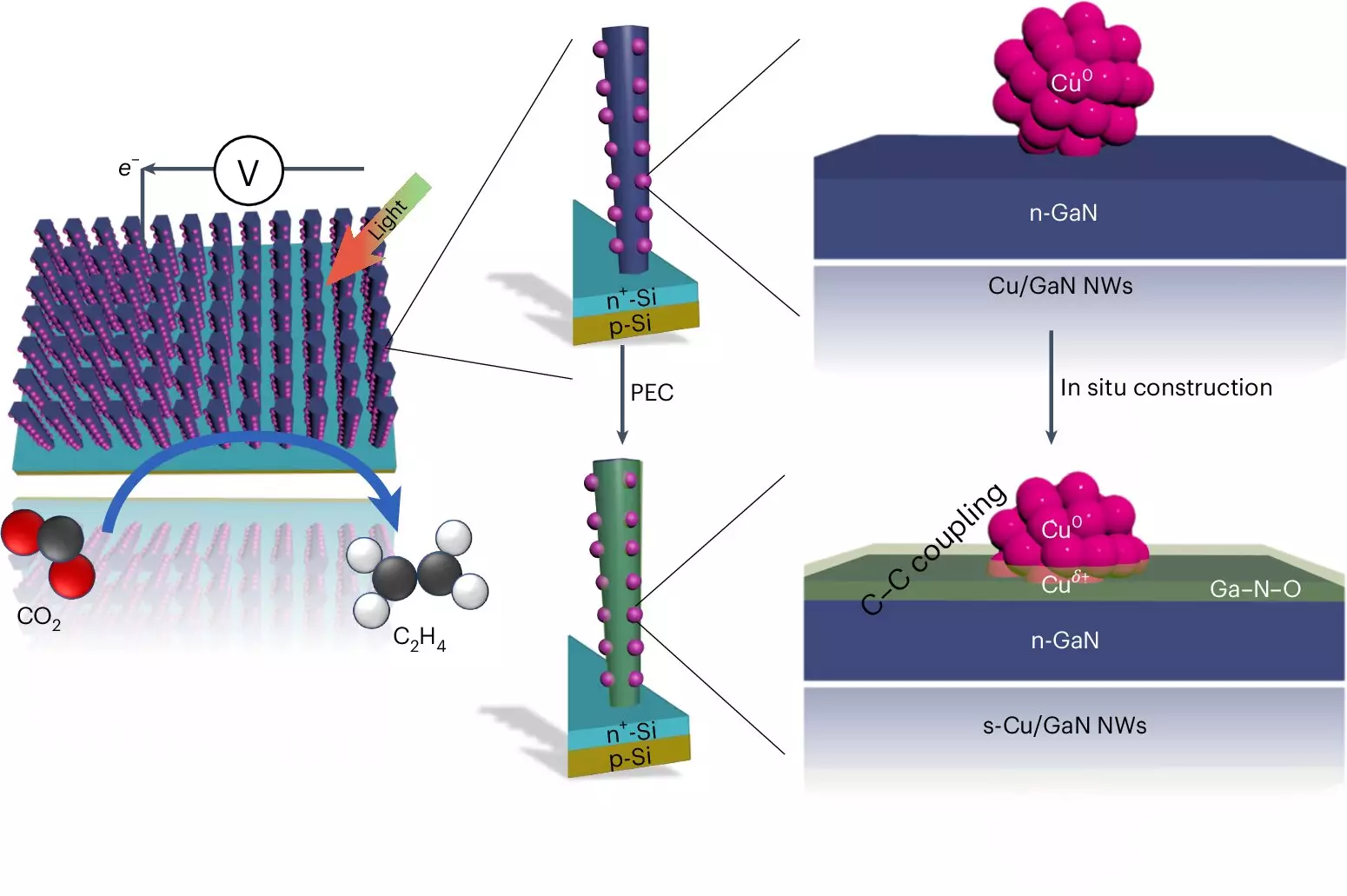
At the heart of this innovative approach lies a sophisticated interplay of materials and chemical reactions. The system utilizes two semiconductor types: gallium nitride nanowires and a silicon substrate. When exposed to intense sunlight, these nanowires absorb energy that catalyzes the splitting of water molecules. This process generates hydrogen while also yielding oxygen, which is simultaneously absorbed to form gallium nitride oxide. Subsequently, copper clusters on the nanowires actively facilitate the necessary chemical reactions.
The process begins when free electrons, energized by sunlight, initiate the splitting of water, liberating hydrogen for the reaction to create ethylene. The unusual effectiveness of this operation is attributed to the cooperative dynamics between the gallium nitride and the copper catalysts. The copper not only binds to hydrogen but also interacts effectively with the carbon atoms derived from CO2, converting them into carbon monoxide—a precursor for ethylene production.
A major hurdle in harnessing CO2 as a fuel source has long been the removal of oxygen from the CO2 molecule, particularly when creating longer carbon chains desired for liquid fuels. The innovative system route addresses this challenge by replacing oxygen with hydrogen in a highly efficient manner, thereby setting the stage for more complex hydrocarbons in the future.
Performance metrics illustrate the impressive capabilities of the Michigan team’s device. The system achieves an ethylene production rate that is reportedly four times higher than its closest competitors. Traditional catalysts, such as combinations of silver and copper, are typically limited in both efficiency and longevity. Their effectiveness wanes after only a few hours, necessitating further innovations.
In stark contrast, the University of Michigan’s device has demonstrated consistent performance across extensive durations, exceeding 116 hours of continuous operation. In additional trials, similar devices have sustained operation for as long as 3,000 hours, a testament to their robust resilience. The longevity of this device is bolstered by the self-healing properties of the gallium nitride oxide when exposed to the oxygen produced during the reaction, further enhancing the longevity of the catalyst.
As the researchers eye future developments, their aspirations extend beyond ethylene production alone. The potential to create other multi-carbon compounds, such as propanol, could revolutionize liquid fuel technologies, thus enhancing the sustainability of transportation systems globally. This transition not only highlights the promise of artificial photosynthesis in creating renewable energy sources but also underscores the vital role of consistent research and technological development in combating climate change.
The University of Michigan’s artificial photosynthesis system represents a significant advance in carbon utilization, potentially reshaping energy consumption and production patterns. By turning waste gases into usable fuels and materials, we take a crucial step towards a greener, more sustainable future. The continuous pursuit of such technological innovations is imperative as we look to mitigate climate change and foster an environmentally responsible economy.

Leave a Reply