The advancement of sustainable technology is more critical than ever, especially in the context of chemical separation processes, traditionally dominated by energy-intensive methods. Recent research efforts from a team at the University of Illinois Urbana-Champaign have unveiled a breakthrough in this field, showcasing a polymer that behaves like an “electric sponge.” This innovative material selectively engulfs specific substances from a solution when stimulated by electrical energy. This novel approach marks a significant pivot towards more eco-friendly practices in chemical synthesis and pharmaceuticals, where precise compound separation is often needed.
Mechanisms Behind Selective Separation
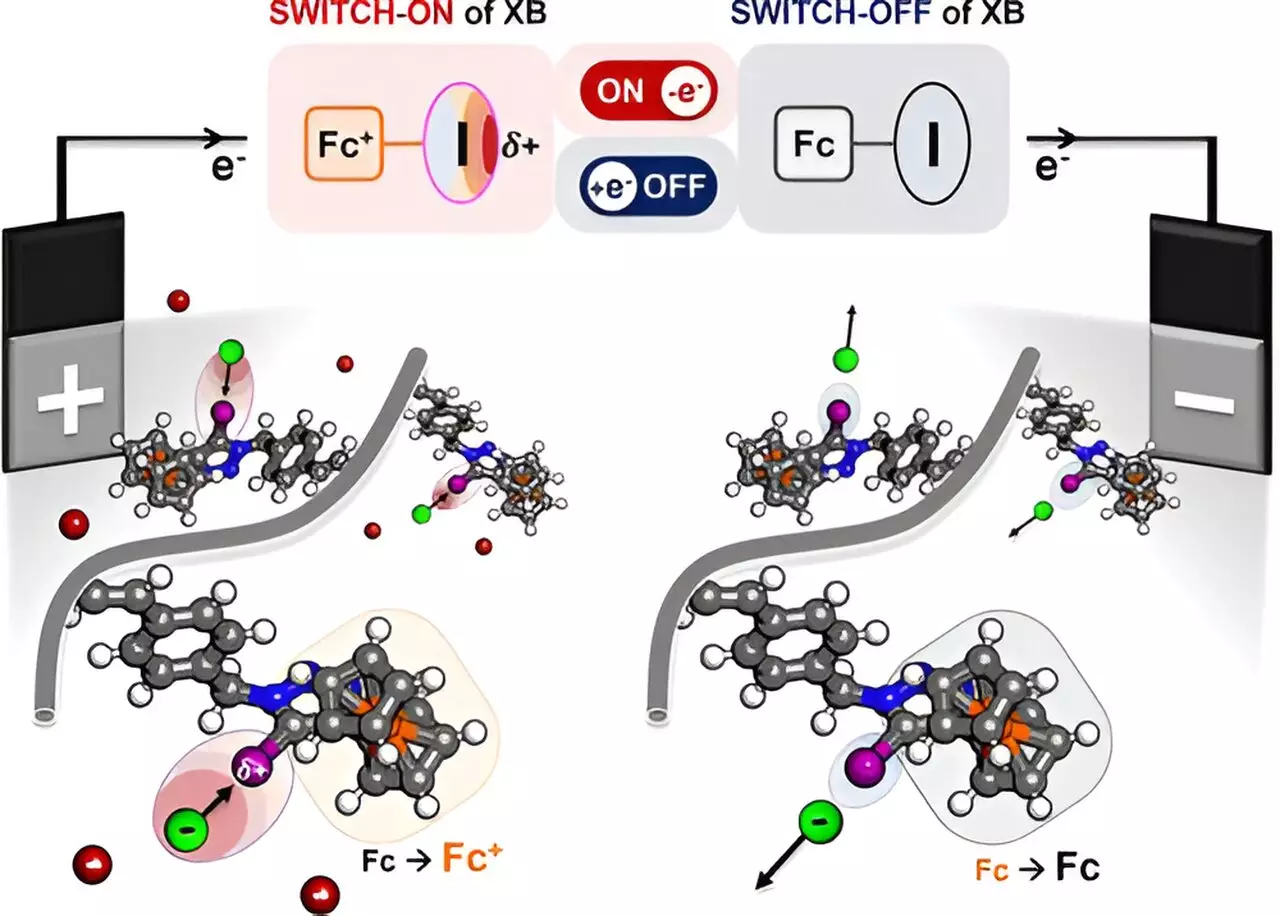
At the heart of this innovation is the concept of halogen bonding, a particular chemical interaction that has previously been underexplored in practical applications. The researchers engineered a polymer embedded with iodine atoms that respond to electrical stimulation, allowing the polymer to evolve in its charge density. Specifically, as electricity is applied, the semiconductor ferrocene in the polymer activates, leading to a marked increase in the positive charge of the iodine atoms. This charge induces an attractive force on target substances, including halides, oxyanions, and various organic molecules.
The significance of this method lies in its selectivity; unlike conventional electrochemical methods, which indiscriminately interact with various ions, this “electric sponge” precisely pulls only the desired ions from a mixture. This level of specificity is crucial in pharmaceutical applications where the purity of compounds is paramount and the elimination of unwanted byproducts is necessary to avoid complications in subsequent reactions.
Comparative Analysis with Existing Methods
Traditionally, methods for chemical separation, such as distillation and membrane filtration, raise concerns primarily due to the substantial material waste they engender. The classic separation processes often require high temperatures or extensive filtration systems that consume large amounts of energy. In stark contrast, the electrochemical method developed by this research team stands to minimize waste generation substantially. By operating at room temperature and drawing on sustainable electricity sources, this new technique not only enhances efficiency but also posits a more environmentally friendly alternative to widespread industrial practices.
The journey of developing this polymer was not without rigorous testing and validation. The researchers conducted various experiments to confirm the efficacy of halogen bonding in the polymer, employing sophisticated techniques such as nuclear magnetic resonance (NMR) and Raman scattering. These validations demonstrate a mindful approach to scientific inquiry, ensuring the robustness of their findings. Collaboration with computational chemists further enriched the endeavor, as simulations helped elucidate the underlying mechanisms that enable the polymer’s functionality, providing a comprehensive understanding of the processes involved.
Looking ahead, the implications of this research are transformative, not only for laboratory applications but industrial practices as a whole. The team, led by Professor Xiao Su, is now focused on refining the technology to enhance its practical applicability. The next phase includes scaling up the production capabilities while ensuring the purity and efficiency of the separation process. Exploring strategies like cascade models could greatly improve the overall effectiveness of the continuous electrosorption systems being developed.
Moreover, transferring these findings from laboratory settings to real-world applications presents challenges and necessitates further investigation. The team’s goals include conducting tests outside the controlled environment of their laboratories to assess the performance stability and adaptability of the polymer in varying conditions that reflect those found in typical industrial scenarios.
This research exemplifies a potent merging of theoretical chemistry and hands-on engineering, with far-reaching implications for the future of chemical separation. By creating a polymer that disaggregates specific substances through a sustainable electrochemical approach, the University of Illinois team has paved the way for a potentially ground-breaking methodology in green chemistry. As we transition towards more sustainable chemical processes, innovations such as this provide a glimpse into a future where industries can meet their material needs with minimal environmental impact, enhancing both purification processes and ecological responsibility.

Leave a Reply