The world of chemical synthesis is continuously evolving, driven by the need for more efficient and environmentally friendly processes. Traditionally, many chemical reactions occur in organic solvents, which, while effective, come with significant environmental drawbacks. More than 80% of the waste generated from these chemical processes can be traced back to the use of organic solvents, raising concerns about their disposal and the overall impact on the ecosystem. However, innovative research from the Indian Institute of Science (IISc) is challenging this status quo by developing a novel surfactant derived from agricultural waste, which promises to revolutionize the way industries approach chemical synthesis.
The reliance on organic solvents poses several challenges, particularly for synthetic chemists. Substrates and catalysts often exhibit sensitivity to water, leading to unwanted side reactions when water is involved. This inherent difficulty has forced chemists to resort to more toxic alternatives, exacerbating the environmental burden associated with chemical manufacturing. The pollution produced from improper disposal of these solvents is alarming, necessitating urgent attention from the scientific community and industrial sectors alike.
Given India’s position as the second largest producer of cashew nuts worldwide, utilizing agricultural byproducts serves a dual purpose: it addresses waste management and provides a sustainable source of raw materials for chemical research. This intersection of sustainability and innovation is at the heart of the research conducted by a team at IISc’s Department of Inorganic and Physical Chemistry.
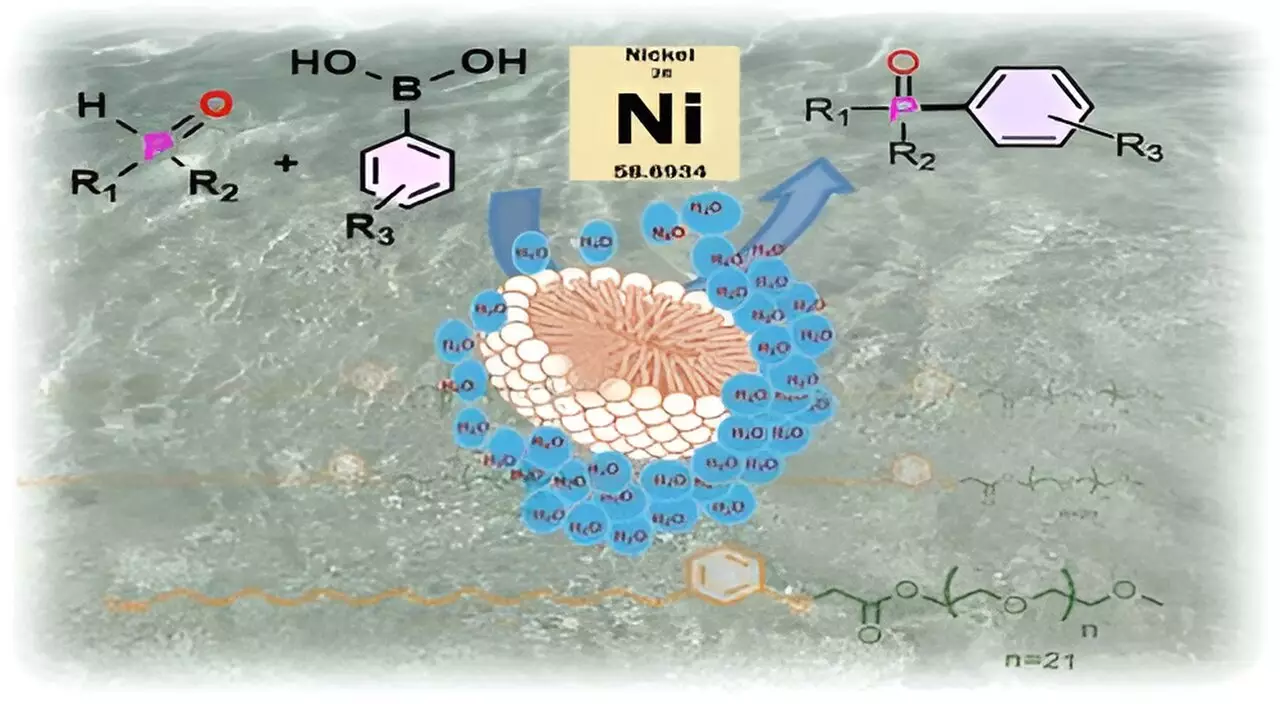
The breakthrough surfactant, aptly named CNSL-1000-M, is derived from cashew nut shell liquid (CNSL), an underutilized agricultural waste resulting from the processing of cashew nuts. The surfactant was synthesized through a meticulous process that combined cardanol—a hydrophobic component extracted from CNSL—with m-PEG, a hydrophilic polymer. This unique combination allows the surfactant molecules to self-assemble into micelles when introduced to water.
In simple terms, these micelles act as protective pods that house sensitive substrates and catalysts, isolating them from the aqueous environment while still engaging in chemical reactions. By shielding these components from the effects of water, such as hydrolysis or unwanted side reactions, researchers can ensure a higher yield of desired products compared to traditional methods.
The concept of micellar catalysis mimics biological systems, where many enzymes utilize hydrophobic pockets for efficient substrate conversion. This biological analogy provides not only a novel methodology but also insight into optimizing chemical reactions that are crucial for various industries.
The IISc team specifically tested CNSL-1000-M in catalyzing the formation of carbon-phosphorus bonds, a reaction vital for synthesizing several high-value compounds, including anticancer agents and materials for organic light-emitting diodes (OLEDs). Remarkably, reactions using the surfactant demonstrated an 80% improvement in product yield when compared to conventional organic solvent methods and showed a 30% increase in yield when stacked against existing surfactants.
This research opens the door to substantial cost savings for industries by reducing the reliance on expensive catalytic materials like palladium and promoting reactions that occur at lower temperatures. Such advancements are not only economically advantageous but align with global efforts to foster sustainable practices in chemical manufacturing.
As the researchers from IISc aim to collaborate with industry players, the potential for transitioning to eco-friendly micellar technology looks promising. The overarching goal is to replace toxic organic solvents with this innovative water-based approach, thereby substantially reducing hazardous waste and environmental impact.
Looking ahead, the team is committed to further investigating micellar chemistry, seeking to broaden its applications and facilitate its industrial adoption. The implications of such research extend beyond just chemistry; they represent a shift towards more responsible manufacturing practices in an era where sustainability is more critical than ever.
The synthesis of CNSL-1000-M offers a compelling narrative of how agricultural waste can be repurposed to create innovative solutions, setting a precedent for future endeavors in sustainable chemical synthesis. This research exemplifies a pivotal step towards environmentally friendly practices that could redefine the landscape of the chemical industry.

Leave a Reply