Carboxylic acids, characterized by their functional group (-COOH), represent a vital class of organic compounds with far-reaching applications, particularly in pharmacology. Drugs like aspirin and ibuprofen owe their effectiveness in part to the unique properties conferred by carboxylic acids. Their ability to interact with biological targets makes them indispensable in medicinal chemistry. However, enhancing their properties often requires the introduction of fluorine atoms, a task that has historically been fraught with challenges due to complex synthesis procedures.
Challenges in Fluorination
The incorporation of fluorine into these molecules is not simply a matter of swapping atoms; it necessitates intricate multi-step synthetic routes that can be tedious and resource-intensive. Traditional methods often face significant hurdles, particularly in the activation of carbon-hydrogen bonds and in establishing carbon-fluorine linkages. The hurdles associated with these processes can stifle innovation and slow down the development of new pharmaceuticals, which is why the recent breakthrough reported by researchers from the Otto Diels Institute of Organic Chemistry is noteworthy.
A Breakthrough in Synthesis
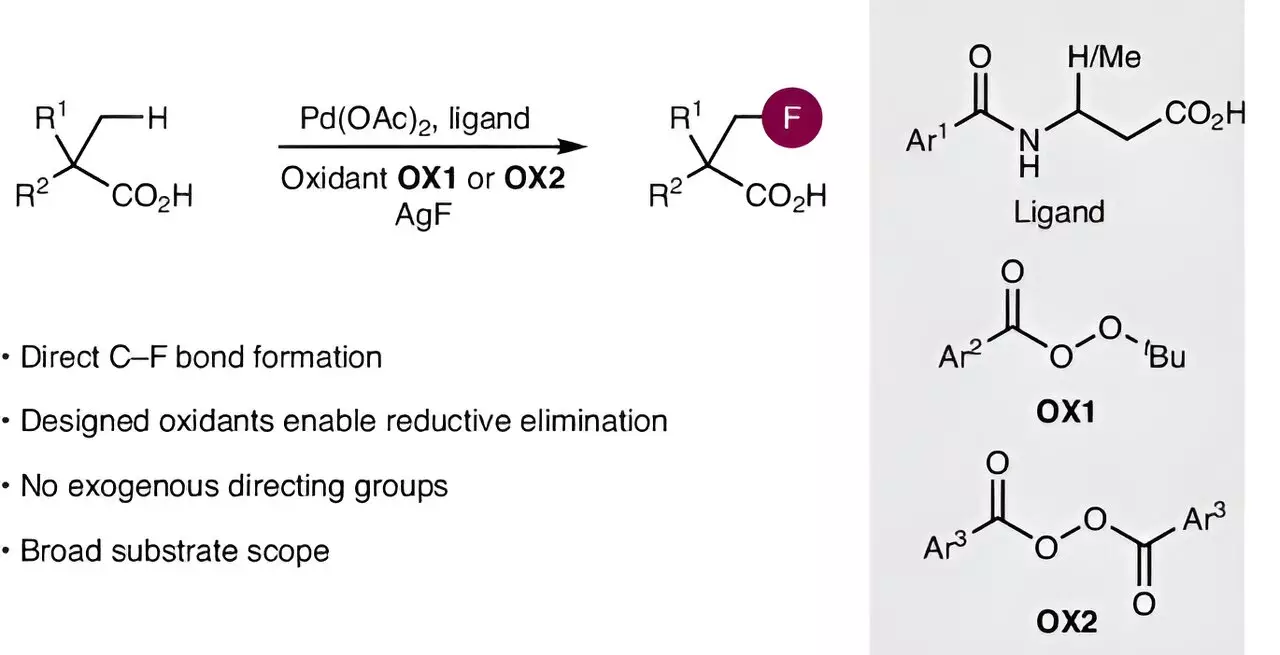
In a landmark study recently published in *Nature Synthesis*, an international team has unveiled a novel method to streamline the fluorination of aliphatic carboxylic acids. Led by Professor Manuel van Gemmeren, the researchers address the multifaceted challenges involved with remarkable efficacy. Their strategy revolves around two main components: activating notoriously inert carbon-hydrogen bonds and establishing stable carbon-fluorine connections. Their pioneering efforts leverage advanced palladium catalysts that have been fine-tuned for this specific application, setting the stage for a significantly simplified approach to synthesis.
The Innovatory Approach
One of the most compelling aspects of the new method is how it diverges from traditional strategies. By developing an innovative oxidizing agent, the team enables a selective formation of the carbon-fluorine bond, an accomplishment that not only overcomes previous limitations but also opens up new avenues for chemical adaptations. According to Sourjya Mal, the first author of the study, the synergy stemming from the refined catalyst design coupled with the newly conceived oxidant facilitates reactions that were previously deemed unreachable. This innovative approach suggests a transformative potential for synthetic methodologies across the field, inviting researchers to reconsider established techniques.
The Implications for Pharmaceutical Research
The implications of this research extend far beyond mere academic interest. As Professor van Gemmeren articulates, the ability to efficiently incorporate fluorine atoms into carboxylic acids could revolutionize drug design and development. The growing importance of fluorinated compounds in pharmaceuticals cannot be overstated; they often enhance the efficacy, bioavailability, and stability of drugs. The researchers’ method thus stands to unlock new therapeutic possibilities, dramatically pivoting the landscape of medicinal chemistry.
The groundbreaking work from the Otto Diels Institute offers not only a practical solution for chemists but also a glimpse into a future where advancements in synthetic methods accelerate drug discovery and development. The potential applications of such innovations herald an exciting time for the field, bridging chemistry and pharmacology in profound ways.

Leave a Reply