In recent years, solar energy has become one of the fastest-growing renewable energy sources in the United States, with advancements in technology leading to more efficient conversion of sunlight into electricity. However, there is also a growing interest in utilizing light to drive chemical reactions. The chemical industry plays a crucial role in producing everyday consumer and industrial products, requiring a significant amount of energy from non-renewable sources to convert chemicals into essential materials such as gases, plastics, pharmaceuticals, and more. According to the 2022 U.S. Department of Energy Industrial Decarbonization Roadmap, the chemicals and petrochemicals sectors contribute to about 40% of all industrial energy consumption and emissions in the U.S.
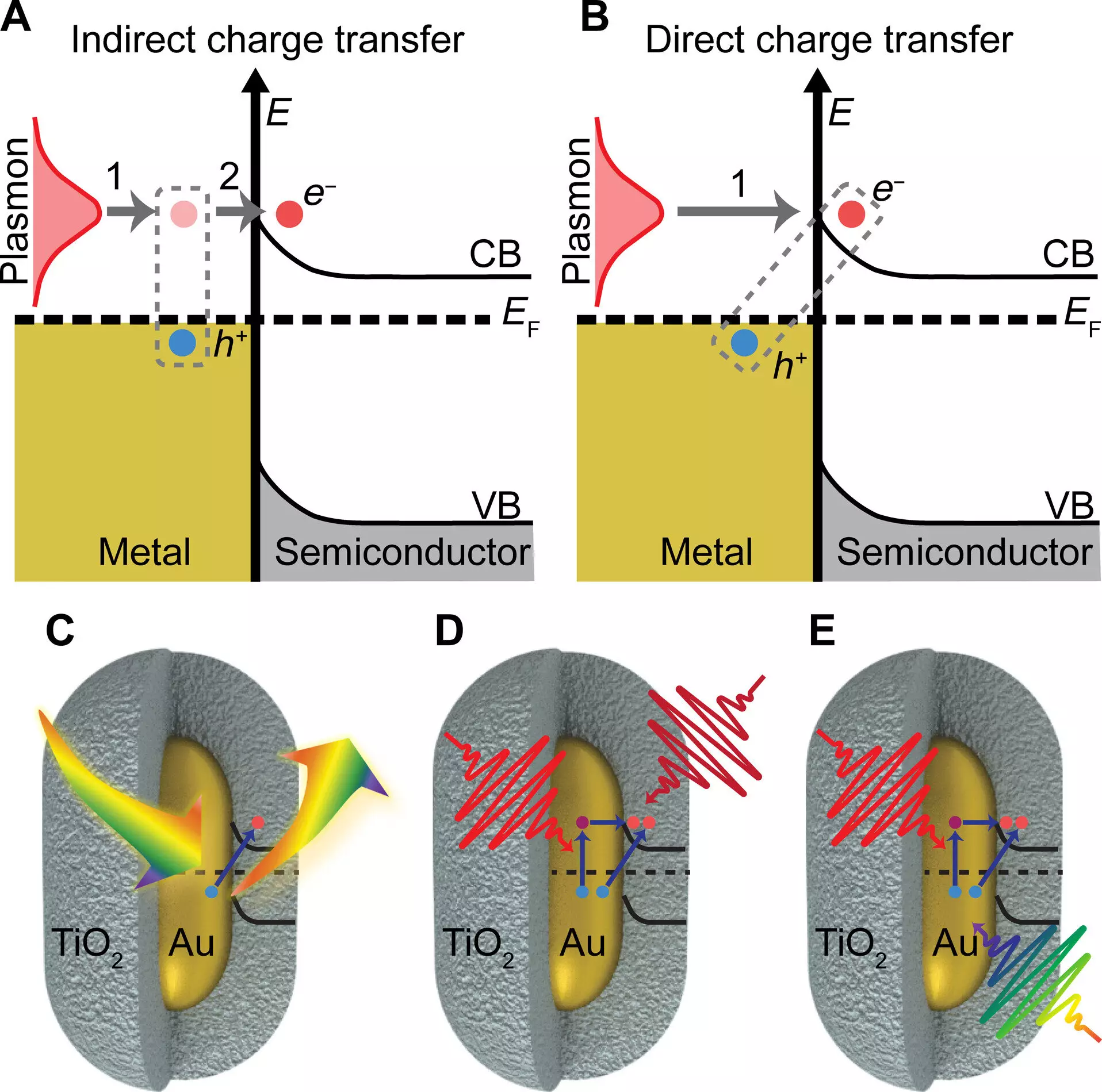
Researchers at the University of Illinois Urbana-Champaign, led by chemistry professor Christy Landes, have been exploring new ways to conduct chemical reactions using light as a catalyst. Their collaborative efforts have uncovered a novel mechanism of charge transfer that not only operates at a significantly faster pace than traditional methods but also doubles the overall charge transfer efficiency. Published in Science Advances, their study focuses on leveraging the unique properties of plasmonic gold nanoparticles, which are extremely small at just 1/1000th the width of a human hair.
The researchers discovered that by integrating plasmonic gold particles with a semiconductor material consisting of titanium oxide shells, they could enhance charge transfer efficiency. When exposed to light, the gold nanoparticles exhibit a high absorption rate, generating collective electronic oscillations when coupled with surface plasmons. This phenomenon enables more efficient charge transfer to the semiconductor material, significantly improving the potential for light-harvesting technologies to generate currents or drive chemical reactions.
One of the key findings of the study was the confirmation that exciting the plasmon with light leads to a boost in charge transfer efficiency to the semiconductor material. Professor Stephan Link, co-author of the paper, emphasized the importance of the plasmon’s role in facilitating charge separation. By absorbing light of a specific color that matches the gold particles, the researchers observed a substantial increase in charge transfer efficiency. This enhanced transfer process acts as a shortcut to achieving the charge-separated state, bypassing the potential heating effect caused by light absorption.
The research team’s findings shed light on a long-neglected process known as chemical interface damping of plasmons, a theory proposed in the 1990s. This discovery highlights the significance of understanding the chemical interactions at the interface between plasmonic nanoparticles and semiconductors. By incorporating various imaging and spectroscopy techniques, the researchers were able to confirm their results and provide new insights into the mechanisms driving plasmon-induced charge transfer.
The innovative research conducted at the University of Illinois Urbana-Champaign offers a promising outlook for revolutionizing chemical reactions using light-absorbing gold nanoparticles. By leveraging the unique properties of plasmonic particles and semiconductor materials, the study opens up new possibilities for more efficient and sustainable methods of driving chemical processes. With continued advancements in this field, we may soon witness a transformation in how we harness solar energy to power essential chemical reactions, paving the way for a cleaner and more sustainable future.

Leave a Reply