Ice is a remarkable state of matter, enigmatic in its interactions with the environment. We often take for granted the roles ice and water play in our daily lives, yet the dynamics at play between them are crucial in myriad contexts, from climate science to culinary delights. Recently, groundbreaking research from Kobe University and the Institute for Molecular Science has taken a significant step toward decoding this complex relationship. This study provides unprecedented insight into the interface between ice and liquid, directly revealing how they interact—a feat that could have far-reaching implications for both scientific understanding and practical applications.
A Leap Forward in Ice Research Methodology
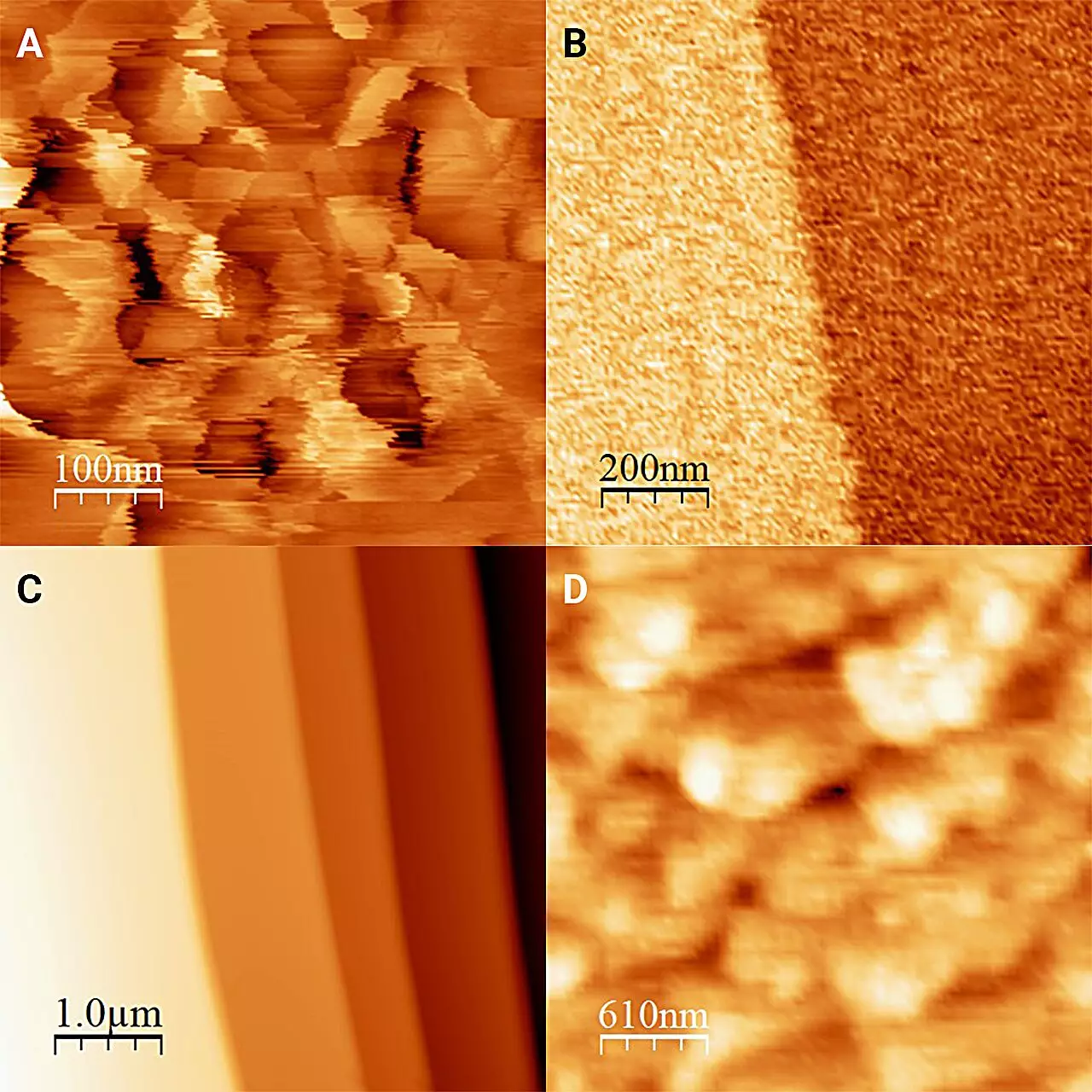
The ingenious approach employed by researchers led by Onishi Hiroshi demonstrates the power of innovation in scientific inquiry. By immersing ice in antifreeze and employing a refrigerated microscope, the team was able to stabilize the interface and render observations that were previously deemed unattainable. The dual challenges presented by the phase transitions of ice and water had long stifled direct observation, making it nearly impossible to investigate their interface. Yet, through meticulous experimentation and iteration, the researchers succeeded in not only stabilizing the ice structure but also capturing its precise characteristics. Their determination to overcome these hurdles encodes a vital lesson about perseverance in the face of scientific obstacles.
Unraveling the Mysteries of Frost Pillars and Flat Surfaces
What the researchers uncovered about the structure of ice at its boundary with liquid solutions was particularly revelatory. The formation of “frost pillars”—20 nanometers in height—was previously an established concept; however, when subjected to antifreeze, the ice surface presents as unexpectedly flat, with only minute steps rising to a molecular height. This radical transformation challenges existing beliefs about ice’s morphology and confirms that ice isn’t a static entity, but instead, a dynamic one capable of changing state. The findings suggest that the interaction between different liquids and the ice can lead to altercations in its physical structure, providing a rich field for further inquiries.
The Role of Antifreeze and Comparisons Among Liquids
In exploring various liquids, particularly alcohols like 1-octanol, the researchers revealed an unexpected variability in the appearance of the ice surface. Even as these liquids were similar in many chemical attributes, the differences in their interactions with ice reinforced the necessity for meticulous, direct measurements. This detail is crucial; it emphasizes not just the unique qualities of ice itself but also highlights the broader implications concerning our environmental interactions—particularly in a world facing climate change and shifting temperature conditions. Thus, understanding these subtle differences may also be integral in predicting larger ecological phenomena.
Strength Beyond Expectation: The Hardness of Ice Uncovered
An equally intriguing aspect of the study involved evaluating the hardness of ice in contact with 1-octanol. The realization that ice may be considerably harder than previous estimation models suggested is a remarkable breakthrough. This newfound understanding could challenge existing frameworks in materials science, possibly shifting how we might approach the engineering of materials that rely on icy substrates or that simulate icy conditions. The practical implications could extend to industries such as cold climate construction, snow and ice management systems, and perhaps even recreational activities on ice.
Looking Ahead: The Promise for Ice Research
The findings from this research are merely the first step in a larger journey. Onishi and his team not only hope to stimulate further investigation into the ice-liquid interface but are also setting ambitious objectives for their future work. Their aspiration to enhance the microscope’s resolution to the infinitesimal scale of individual water molecules speaks to a broader scientific aim: to deepen our understanding of the foundational elements of nature. This pursuit could open new avenues across numerous disciplines, from nanotechnology to meteorology, giving rise to insights that could shape our future.
The opening of this dialogue about the intricate relationship between ice and its liquid counterpart is essential for progressing our grasp of natural phenomena. As we look towards the future, the importance of engaging actively with this research cannot be overstated. Stepping into the unknown is what drives science forward, and this study lays a fascinating groundwork for future explorations.

Leave a Reply