The quest for high-performance catalysts has always been a central pursuit in the fields of chemistry and materials science, especially regarding energy conversion technologies like water splitting. The oxygen evolution reaction (OER) plays a pivotal role in these processes. Traditionally, noble metals such as iridium have been utilized for their outstanding catalytic properties, but their high cost and scarcity present significant barriers to the widespread adoption of these technologies. Consequently, researchers have been exploring alternatives that are both economically viable and effective under various conditions, notably acidic environments where OER is particularly challenging.
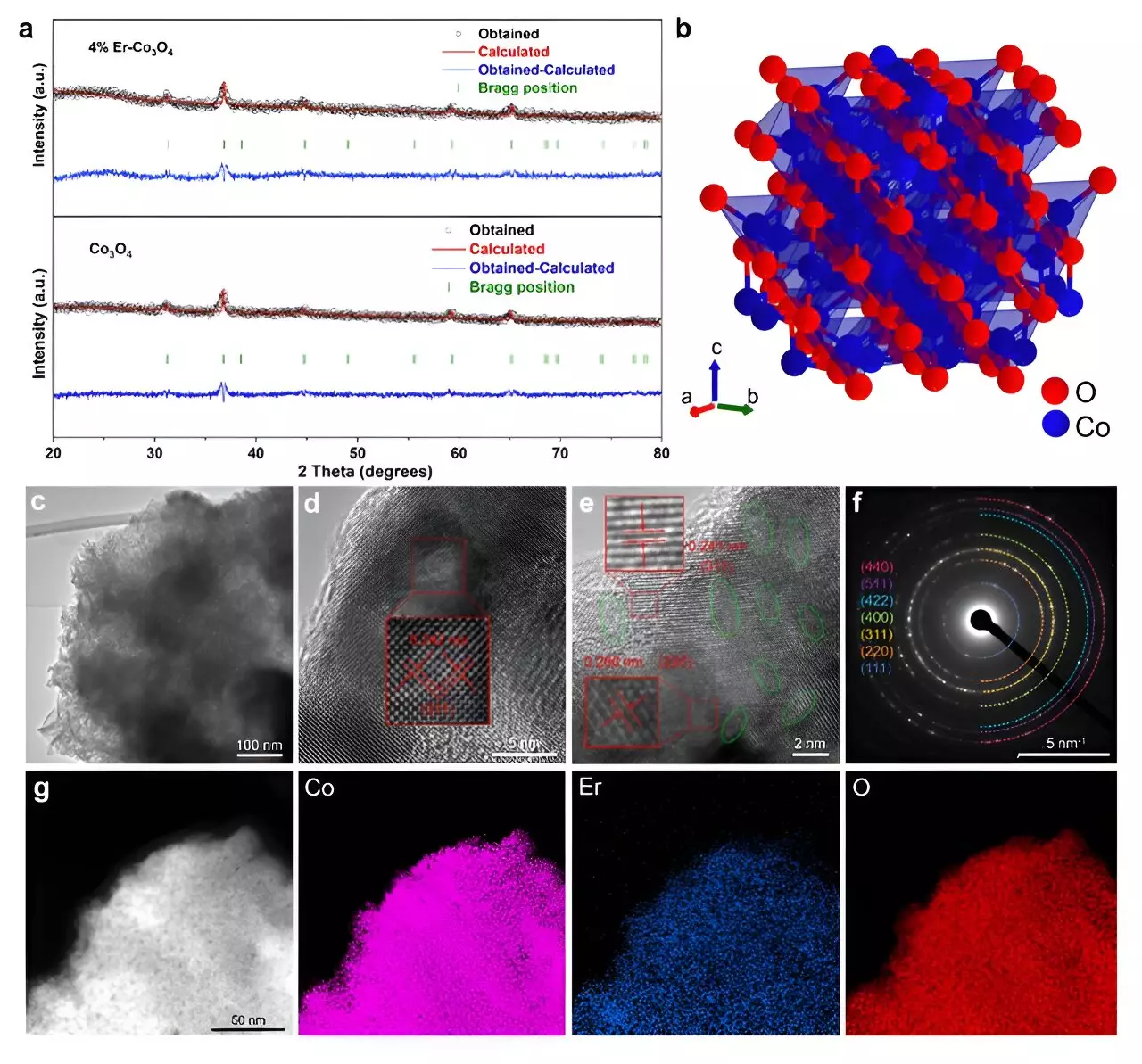
Recent advancements have spotlighted the work of a research team who integrated erbium (Er), a rare earth element, into cobalt oxide (Co3O4) to develop an innovative doped electrocatalyst. This breakthrough not only promises enhanced efficiency in OER but also positions itself as a viable competitor to existing noble metal catalysts.
The Breakthrough: Erbium-Doped Cobalt Oxide
The introduction of erbium into the Co3O4 matrix represents a significant advancement over the limitations of conventional cobalt-based catalysts. The study, highlighted in ACS Catalysis and recognized as an Editors’ Choice, showcases that the inclusion of just 4% of Er substantially improves the catalytic performance of the cobalt oxide catalyst, demonstrating an astonishing overpotential of merely 321 mV at 10 mA cm², with stability exceeding 250 hours.
The significance of this development lies not only in the performance statistics but also in its practical implications. By enhancing the effectiveness and durability of non-precious metal catalysts, this research could catalyze broader adoption of sustainable energy technologies by reducing costs and reliance on scarce materials.
A deep dive into the mechanics of the catalyst reveals how erbium doping plays a transformative role in the structural and electronic properties of Co3O4. The research employs a combination of advanced analysis techniques, including microkinetic modeling and density functional theory (DFT) calculations, to elucidate the reasons behind the improved performance of the Er-doped catalysts.
Doping with Er facilitates the creation of more active sites within the crystal structure by altering the ratio of Co3+ to Co2+ ions, a critical parameter for catalysis. This adjustment promotes the formation of oxygen vacancies, accelerating the OER process. According to the research team, the catalyst can be likened to a roadway where erbium acts as a traffic enhancer, introducing additional lanes that permit increased flow of ‘traffic’—in this case, the essential oxygen intermediates needed for the reaction.
Moreover, in situ Raman spectroscopy pinpointed the oxygen vacancies’ location within the Co3O4 structure, confirming that these vacancies are crucial for generating necessary reaction intermediates—essential components for driving OER effectively.
While the results of this study present a compelling case for erbium-doped cobalt catalysts, it is essential to recognize the ongoing challenges that face the field of electrocatalysis. The stability of catalysts under operational conditions remains a concern; however, the research demonstrated that the modified Co3O4 retains its properties significantly longer than conventional catalysts.
Moving forward, the research team anticipates further exploration of non-precious metal doping materials to develop electrocatalysts that not only excel in performance but are also sustainable and readily available. This represent a promising step in addressing both economic and environmental challenges associated with precious metals in energy conversion technologies.
The implications of these findings are profound. By paving the way for cost-effective and efficient catalysts, the erbium-doped Co3O4 provides a promising avenue for enhancing the viability of water splitting technologies. As the global community increasingly seeks sustainable solutions to energy generation and consumption, innovations such as this one could be integral in the ongoing transition toward renewable energy sources.
The research on erbium-doped cobalt catalysts epitomizes how material science can intersect with practical applications to propel advancements in energy technology. By adopting such innovative approaches, we not only seek to improve catalytic processes but also strive toward a more sustainable energy landscape.

Leave a Reply