Polypropylene is deeply intertwined with our daily lives, appearing in an array of products ranging from food containers to medical devices. This wide usage raises the urgency for efficient and sustainable production methods for propylene, the key chemical used in creating polypropylene. As the demand for polypropylene continues to soar, identifying more effective methodologies for producing propylene is critical. The conventional processes are energy-intensive and heavily reliant on precious metals, casting shadows on their ecological viability. The recent research conducted by the U.S. Department of Energy (DOE) presents promising advancements that could revolutionize propylene production and, consequently, the manufacturing of polypropylene.
Addressing Environmental Concerns with Innovative Catalysts
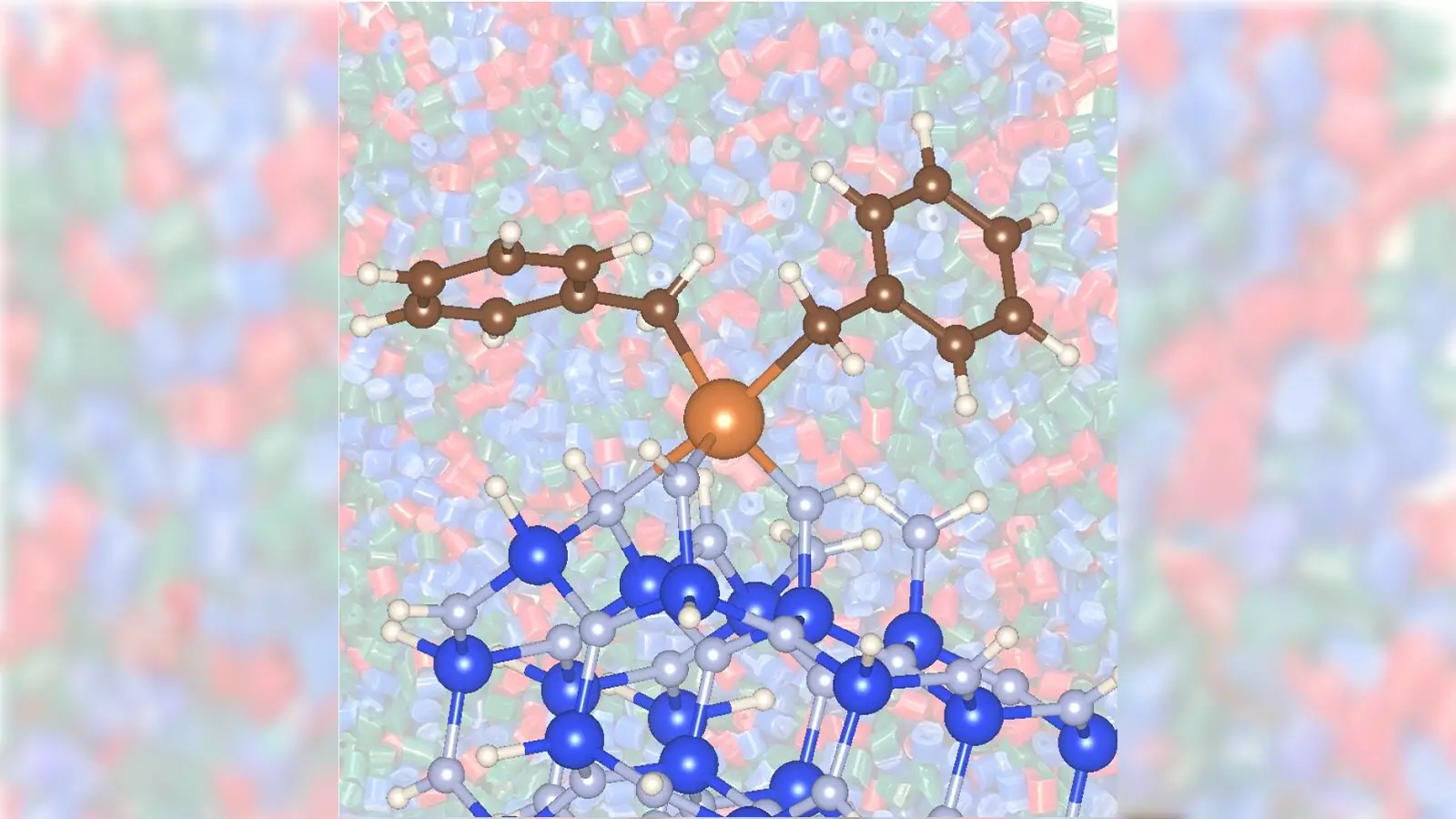
Traditional methods for converting propane into propylene primarily involve metal catalysts such as chromium and platinum. These metals expedite the chemical process but come at a cost—both financial and environmental. The necessity for high operational temperatures inevitably leads to substantial energy consumption and heightened greenhouse gas emissions, contributing significantly to climate change. Scientists at Argonne and Ames National Laboratories have taken a bold step to alleviate these concerns by investigating the efficacy of using zirconium with silicon nitride as a catalyst. Their findings suggest that this combination not only promotes a faster reaction but also reduces energy consumption when compared to conventional catalyst options.
Interestingly, the switch to a zirconium catalyst coupled with silicon nitride has shown promising results, demanding a lower catalytic temperature of approximately 842 degrees Fahrenheit compared to the traditional 1,022 degrees. This substantial reduction not only decreases energy usage but also minimizes carbon dioxide emissions—a crucial stride in battling environmental degradation. Given that nearly 80% of greenhouse gas emissions emanate from the U.S., advancements that promise lower emissions are not just beneficial but necessary.
The Role of Advanced Characterization Techniques
A significant part of this groundbreaking study involved cutting-edge techniques to delve deeper into catalyst interactions. Utilizing Argonne’s Advanced Photon Source (APS), researchers employed X-ray absorption spectroscopy to meticulously assess how the zirconium catalyst behaves in conjunction with the silicon nitride support, as opposed to traditional oxide materials. Such innovative approaches provided invaluable insights into the distinguishing properties of the zirconium and silicon nitride combination. This meticulous attention to material characterization underscores the potential for further advancements in catalysis.
Additionally, insight into the structural dynamics of the zirconium/silicon nitride catalyst was provided by Frédéric Perras, a scientist who utilized nuclear magnetic resonance techniques. By examining how silicon nitride interacts with metal sites, researchers have uncovered layers of complexity that could inform future studies and enhancements in catalysis. The amalgamation of experts from various disciplines is a testament to the collaborative spirit driving this research. As Delferro aptly notes, no single researcher can encompass the range of expertise necessary to achieve such innovations; it is the collective effort that brings about significant breakthroughs.
Broadening Horizons: Implications for Future Catalytic Processes
The implications of this research extend beyond the immediate benefits of more efficient propylene production. The study opens the door to exploring the reactivity of other transition metals when supported by silicon nitride. This exploration holds potential for a myriad of reactions, spanning multiple industries and applications. Kaphan’s assertion that this could serve as a foundation for future studies on nitride-supported metals exemplifies the research’s broader applicability and highlights a pathway toward reducing reliance on precious metals.
The zealous exploration of nontraditional catalytic systems reflects an exciting shift in the field of materials science. Moving away from conventional frameworks not only addresses economic concerns—due to the lower cost of alternatives—but also caters to an urgent need for sustainable practices in the chemical industry. This study encapsulates a broader paradigm evolution in catalysis, fostering an environment ripe for innovation and promise.
Fostering a Sustainable Future
As humanity grapples with the reality of climate change and the depletion of resources, the necessity for sustainable production processes has never been more pressing. The innovative work performed by the researchers at Argonne and Ames serves as a beacon of hope, highlighting that advancement in material science can coincide with environmental responsibility. If the momentum behind such research can be sustained, the future may very well see a paradigm shift in how essential materials like polypropylene are produced—ushering in an era that appreciates both efficiency and ecological integrity.
By prioritizing sustainability in foundational chemical processes, we can pave the way for a future where industrial advancements do not come at the expense of our planet. The real victory lies not merely in the immediate returns of enhanced production efficiency, but in establishing a legacy where innovation and environmental stewardship go hand in hand.

Leave a Reply