The global demand for sustainable energy sources has never been more urgent, pushing scientists to find innovative ways to convert waste materials into valuable fuels. A recent study published in *Nature Catalysis* has shed light on a promising method for synthesizing methanol from carbon dioxide (CO2)—a notorious greenhouse gas. This research, backed by an international collaboration, centers on leveraging electric currents to enhance the efficiency of the transformation process, a significant stride in the quest for cleaner fuels and materials.
Chemists have long been aware of the potential of CO2 recycling but struggled to optimize the conversion processes. The breakthrough lies in using cobalt phthalocyanine (CoPc) molecules arranged on carbon nanotubes— fascinating structures that exhibit unique electrical characteristics. This combination allows a novel approach in which electrochemical reactions can occur, leading to high-efficiency conversion of CO2 into liquid fuels. The implications of this technique extend far beyond just methanol; it opens avenues for creating various chemicals crucial for energy storage and other applications.
A Closer Look at the Mechanism
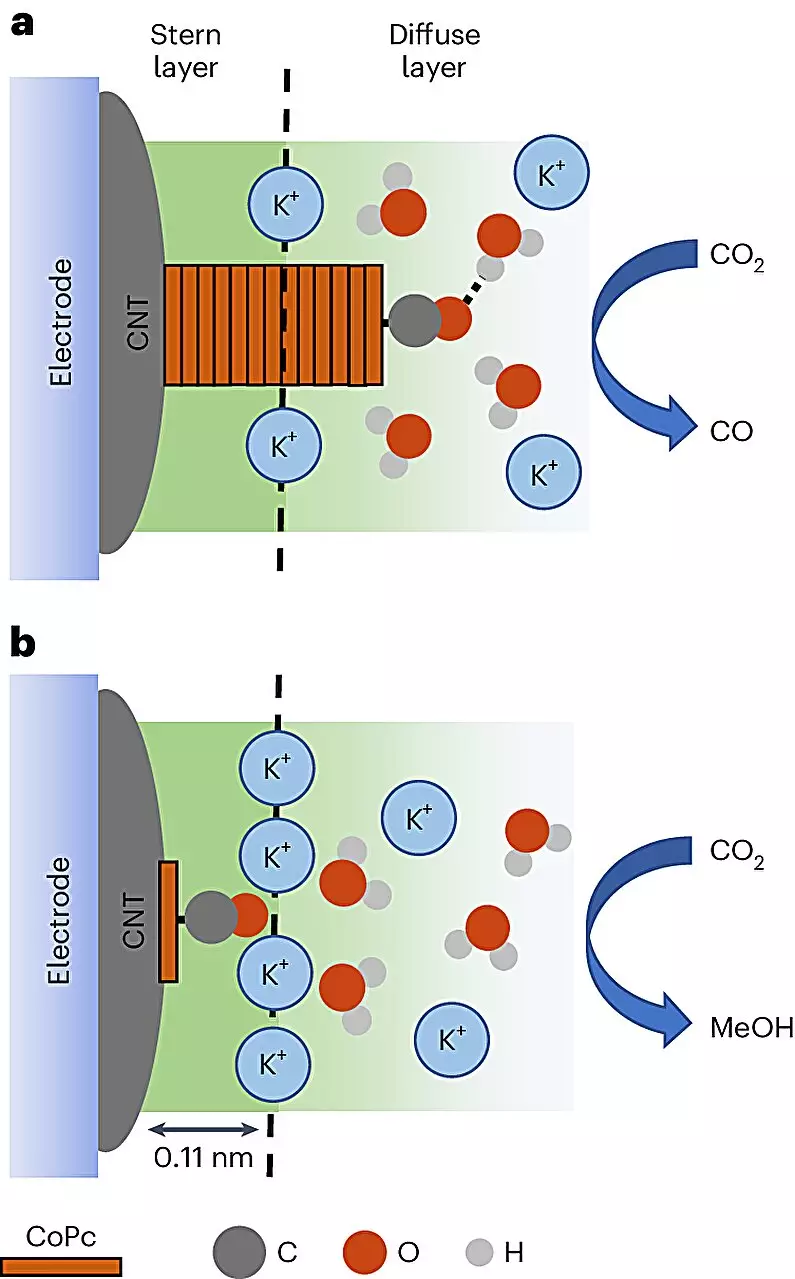
The intriguing aspect of this study is the technique utilized for visualizing the reaction—an advanced method labeled in-situ spectroscopy. For the first time, researchers were able to observe the conversion of CO2 into methanol and the alternative production of carbon monoxide (CO) in real-time. This capability is a game changer in catalysis research, permitting scientists to grasp the subtleties of catalytic efficiency. The findings indicate that the reaction path can significantly shift depending on the environment of the CO2 molecules, enabling researchers to manipulate conditions effectively to maximize the yield of methanol.
Professor Robert Baker, a co-author of the study, emphasized the importance of understanding the catalytic processes at a nuanced level. Until now, the chemistry behind why certain catalysts work better than others has often remained a mystery. With the new insights from this research, much-needed clarity is finally emerging. Baker’s expertise in surface chemistry shines through, demonstrating that subtle adjustments in the distribution of catalysts on the carbon nanotubes can create conditions where CO2 molecules are up to eight times more likely to yield methanol.
The Role of Vibrational Spectroscopy
Leading the charge in this research was Quansong Zhu, who harnessed vibrational spectroscopy to analyze the molecular dynamics involved in the catalytic process. This technique reveals how molecules interact with their environment, a factor that has remained elusive in prior studies. Zhu pointed out the importance of vibrational signatures in differentiating between the reaction landscapes that lead to methanol production versus those that result in less desirable outcomes.
By correlating these observances with the reaction conditions, researchers were able to ascertain which specific environmental factors favored methanol generation. The ability to monitor and manipulate these elements ensures a finer control of the conversion process, paving the way for larger-scale applications in renewable energy technologies.
Unleashing the Potential of Methanol
Methanol, a highly energy-dense fuel, stands out not just for its performance but also for its versatility. The new methods of production detailed in the study could provide a wellspring of renewable methanol that fuels everything from automotive vehicles to planes, making significant strides toward reducing reliance on fossil fuels. Furthermore, there is potential for this methanol to contribute to heating and power generation, thereby broadening its applicability in both domestic and industrial contexts.
Yet, as Baker notes, the journey doesn’t end here. The intriguing interaction between methanol-forming molecules and positively charged ions, known as cations, warrants further investigation. Understanding these interactions could unlock even more efficient catalytic processes, suggesting that the research is just the tip of the iceberg in uncovering the complexities of chemical transformations at a molecular level.
The Future of Clean Energy Synthesis
This cutting-edge research marks a pivotal moment in the field of sustainable chemistry, with implications that extend into various aspects of energy production and environmental integrity. By harnessing the power of waste materials, specifically CO2, and employing advanced techniques for real-time analysis, scientists are inching closer to a future where renewable energy is not just a possibility but a reality.
The excitement surrounding the potential discoveries that could stem from this study is palpable. As the scientific community continues to explore and refine these innovative processes, the hope is to transition toward a more sustainable energy framework, which not only addresses current demands but also sets a foundation for the energy needs of generations to come. The implications of such advancements are not just theoretical but hold a practical promise of transforming how we utilize resources and tackle climate change.

Leave a Reply