The push for advanced energy storage solutions has intensified in recent years, driven by the increasing demand for efficient and sustainable technologies. Among the most promising contenders in this quest are solid-state batteries, which offer the potential for greater power density, enhanced safety, and longer lifespans compared to traditional lithium-ion batteries. However, the integration of lithium and sodium metal anodes remains fraught with scientific hurdles, particularly due to the inherent challenges associated with these highly reactive elements. Understanding the microstructure of these alkali metals is vital for overcoming these barriers and unlocking the full potential of solid-state batteries.
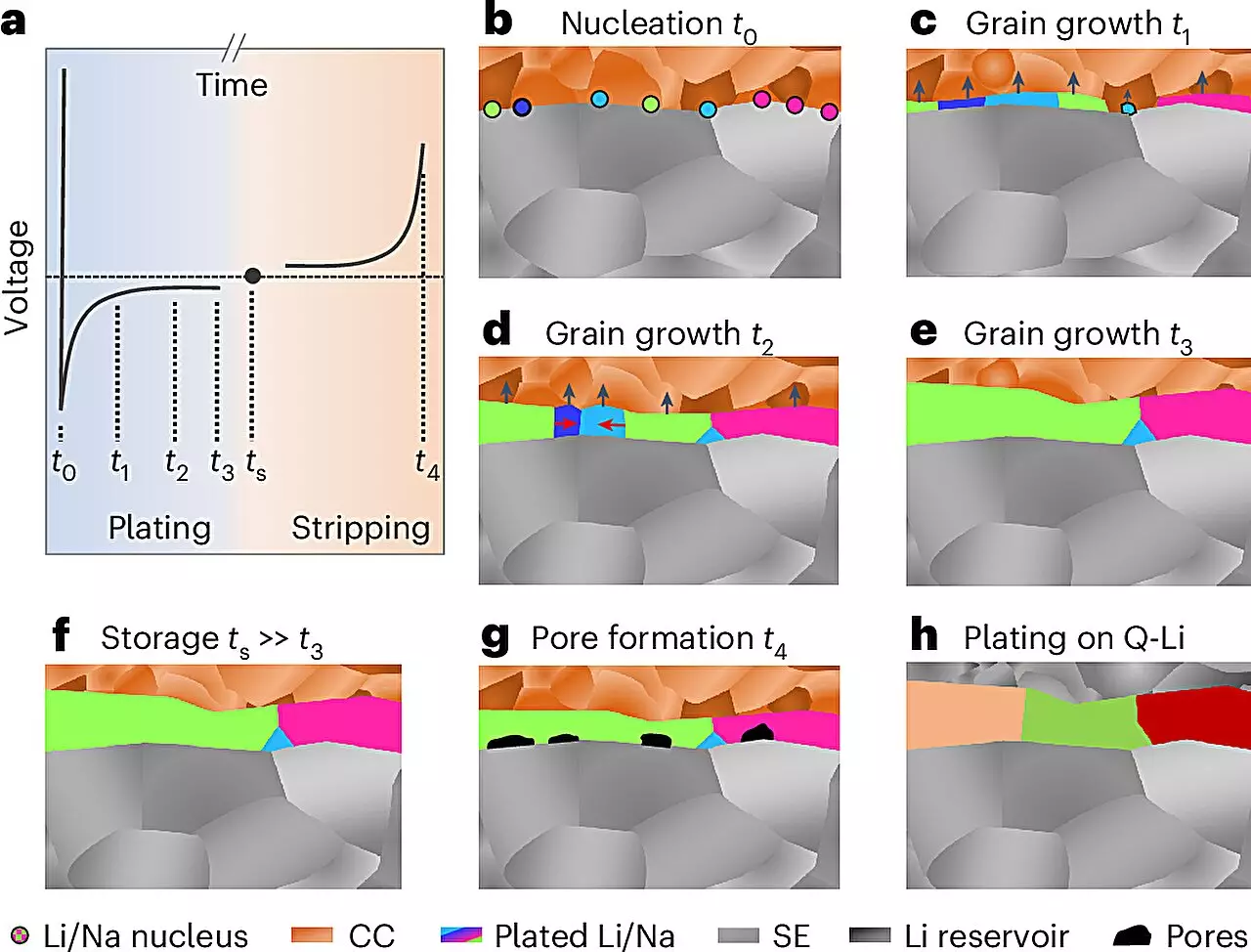
The microstructure of materials plays an integral role in defining their electrochemical characteristics. For metals, a well-defined internal structure can enhance battery performance by improving conductivity, stability, and overall efficiency. While significant research has been conducted around the microstructural properties of many metals, lithium and sodium metals have historically posed unique challenges due to their reactive nature. The formation of reaction layers upon exposure to various environments has rendered it nearly impossible to analyze their microstructures accurately — until now.
A groundbreaking study led by researchers at Justus Liebig University Giessen (JLU), in collaboration with teams from the University of California, Santa Barbara, and the University of Waterloo, has achieved a significant milestone in materials science. For the first time, they successfully elucidated the microstructures of lithium and sodium metal deposited in a battery setting. This research has opened up novel avenues for enhancing the electrochemical properties of these alkali metals, potentially transforming the landscape of solid-state battery technology.
Led by Professor Dr. Jürgen Janek of the Institute of Physical Chemistry at JLU, the research team developed an innovative methodology that incorporates low-temperature preparation and analysis techniques conducted under inert gas conditions. Utilizing electron backscatter diffraction, they achieved detailed imaging of the microstructure in layers of lithium and sodium up to 100 micrometers thick. This finely-tuned process allowed them to not only visualize but also understand the growth mechanisms at play in these alkali metals, yielding surprising findings regarding grain sizes and structural characteristics.
The revelations from this research extend far beyond mere academic curiosity. With a clearer understanding of the microstructure, researchers can now explore methodologies to optimize the performance and safety of lithium and sodium metal anodes. This insight is particularly crucial given that the ultimate goal of developing solid-state batteries involves mitigating the risks of dendrite formation and other structural instabilities that lead to short circuits and compromised battery life.
The collaboration also highlights a significant frontier in sodium battery research. As the scientific community continues to investigate alternative and sustainable energy storage options, sodium-based systems represent an attractive avenue to explore due to sodium’s abundance and low cost compared to lithium. The findings from the JLU research group will undoubtedly inform future advancements in sodium-based technologies, aligning with the broader objectives of the POLiS (Post Lithium Energy Storage) excellence cluster, enhancing both theoretical and practical applications in energy storage.
Despite the promising advancements delineated by this research, several hurdles remain before solid-state batteries can experience widespread commercialization. The tendency of lithium and sodium metals to deform during electrochemical cycles, leading to challenges in charging and discharging processes, must be addressed. Researchers are now faced with developing strategies that allow for metal formation only during the initial charging phase, thereby reducing handling difficulties associated with these reactive materials.
As the field of solid-state batteries continues to evolve amidst extensive global research efforts, the contributions from JLU stand out as pivotal. Professor Janek’s long-standing collaboration with research teams in North America underscores the importance of interdisciplinary work in tackling complex challenges in energy storage technologies. The meticulous approach taken in this latest study underscores the necessity of sophisticated equipment and a collaborative research environment in mapping out the future of solid-state batteries.
The exploration of the microstructural characteristics of lithium and sodium metal anodes marks a significant turning point in solid-state battery research. By unlocking the intricacies of these alkali metals, scientists are paving the way for innovations that could redefine energy storage systems. As researchers continue to unravel the complexities of these materials, the dream of safer, more efficient, and longer-lasting batteries may soon become a reality, propelling us toward a more sustainable energy future.

Leave a Reply