Water pollution remains one of the most pressing environmental issues of our time, impacting ecosystems, human health, and the economy. The increasing levels of contaminants, particularly from industrial waste, call for innovative solutions in water treatment technologies. Researchers at the University of Science and Technology of China (USTC) have recently made a significant stride in this direction by utilizing single-atom catalysts (SACs) in a novel Fenton-like catalytic system, leading to groundbreaking advancements in pollutant degradation efficiency.
Single-atom catalysts are adept at facilitating chemical reactions, delivering potent cleaning power for contaminated water. Traditional applications, however, faced limitations, primarily due to two factors: the sluggish movement of reactants toward the catalyst surface and the excessive amounts of oxidants typically required. This dual challenge has hindered the full potential of SACs in real-world scenarios. Previous endeavours suggested that confining these catalysts could enhance their effectiveness, but the underlying mechanisms remained elusive.
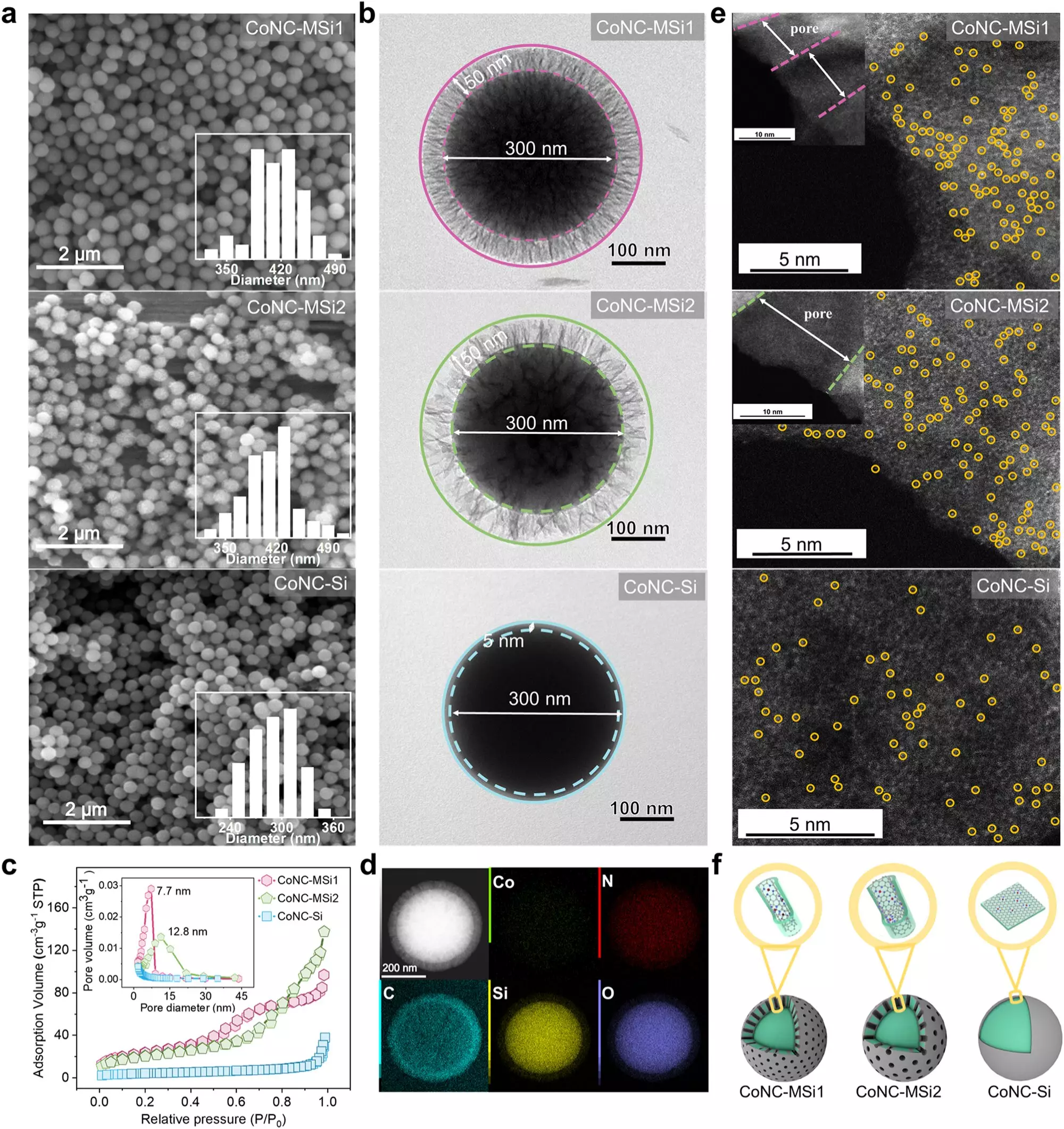
The research team emerged with a transformative approach by employing nanoscopic silica particles to house the SACs. This confinement within minuscule pores proved to be a game-changer, significantly boosting the reaction speeds and optimizing oxidant utilization. Their investigations revealed a pivotal shift in the catalytic pathway—from utilizing singlet oxygen to employing a direct electron transfer mechanism. This shift not only enhances the efficiency of pollutant breakdown but also marks a fundamental conceptual advancement in catalytic chemistry.
The results disclosed by the research team are nothing short of astounding, showcasing a 34.7-fold increase in pollutant degradation rates relative to conventional methods. Further, the efficiency of oxidant usage soared from 61.8% to an impressive 96.6%. Such enhancements are crucial for developing sustainable and economically viable water purification systems. The trial experiments conducted on various electron-rich phenolic compounds revealed not only efficacy but also the robustness of the method across diverse environmental conditions, including real water samples from lakes.
This breakthrough offers profound implications for environmental science and engineering. By illuminating the intricate workings behind nanoconfined SACs, the USTC researchers have paved the way for future innovations in advanced oxidation processes. The findings advocate for a paradigm shift towards low-carbon and highly efficient technologies for water purification, addressing both the short-term needs and long-term sustainability goals in managing water resources.
The recent developments in SACs signify not just an incremental advancement but rather a significant leap forward in the quest for cleaner water. As we grapple with ongoing environmental challenges, such innovative strategies could play a pivotal role in reshaping water treatment practices. The journey towards maintaining the planet’s water resources is fraught with challenges, but with cutting-edge research like this, a cleaner future appears increasingly attainable.

Leave a Reply